
Concept explainers
(a)
Interpretation:
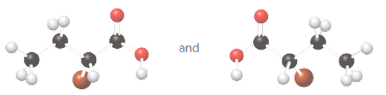
Whether the given pair of molecules are identical, or enantiomers should be determined.
Concept Introduction:
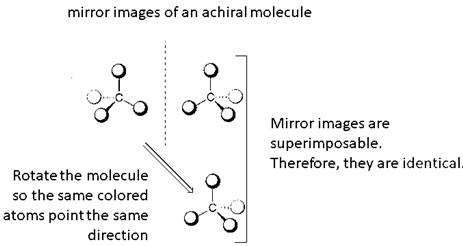
Identical molecules are the ones with no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural arrangement (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
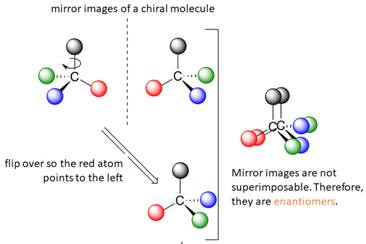
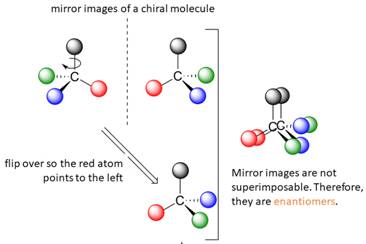
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. Molecules that do not have any chiral centers are called achiral. Identical molecules do not have any chiral centers; therefore, they are achiral.
When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
(b)
Interpretation:
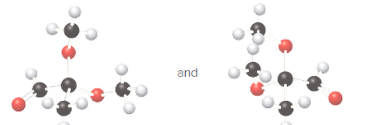
Whether the given pair of molecules are identical, or enantiomers should be determined.
Concept Introduction:
Identical molecules are the ones with the same chemical formula but no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural arrangement (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. Molecules that do not have any chiral centers are called achiral. Identical molecules do not have any chiral centers; therefore, they are achiral.
When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
General, Organic, and Biological Chemistry - 4th edition
- How are the following two compounds related to each other? H H3C KD H3C CH3 a) They are constitutional isomers of each other. O b) O c) H CH3 d) They are enantiomers of each other. They are stereoisomers of each other. They are functional isomers of each other.arrow_forwardsort the compounds into pairs of constitutional isomers, stereoisomers, or identical moleculesarrow_forwardClassifying Compounds as Stereoisomers or Different Conformations Classify each pair of compounds as stereoisomers or conformations: (a) X and Y; (b) X and Z.arrow_forward
- Classify each pair of compounds as either identical, constitutional isomers, stereoisomers, or not isomers.arrow_forwardWhat is the total number of stereoisomers? CI .CI CIarrow_forwardClassify each pair of compounds as constitutional isomers, stereoisomers, identical molecules, or not isomers of each attached otherarrow_forward
- 8. Identify the following sets of compounds as enantiomers, diastereomers, or identical. Relationship Between Compounds (enantiomers, diastereomers, or identical)?arrow_forward. Determine whether the molecules in each pair are the same or enantiomers. H H C CH;CH, a. CH3CH2 l CI CH3 CH3 H H b. H3C HOWCH, Cl но Но CH3 CH3 H CH c. CH3 H HC, CH3 CH3 H;C. H,C || CH2 CH3 CH2arrow_forwardName Consider the 2,3-dibromobutane family of stereoisomers. How are the compounds in each pair related to each other? 1) Identical 2) Enantiomers 3) Diastereomers H3C CH3 H3C CH3 Br Br H H. H3C CH3 H3C CH3 В Br Br H3C CH3 H3C CH3 Br H. H. Br H3C CH3 H3C CH3 Br H. H3C E CH3 H3C CH3 Br Br H. Brarrow_forward
- Part D. Do the two structures A and B of each pair drawn below represent the same molecule, constitutional isomers, or stereoisomers? If A and B are stereoisomers, further classify them as enantiomers or diastereomers. H3C Structure A H3C HO H3C H3C Br T H H3C H HO H3CC H C Br HC H H H H CH₂Br CH₂CH3 H CH₂CH3 CH₂OH OH H HO G G H HO H HO G CH3 CH3 CH3 Br Hallo H H3C H Structure B H3C CH3 Br WING H Br HOCH₂ CH3 CH3 HO H CH₂OH H CH₂CH3 H CH₂CH3 H H H H H H Gim CH3 H OH "H ·GII. CH3 HO. OH OH CH3 CH3 OH Relationship of A and Barrow_forwardFor each pair of compounds, please explain if they are identical. comstitutional isomers, enantiomers, or diastereomers.arrow_forwardWhich of the following statements is/are correct? Br Br Br Br CI 'P' 'R' 'S' .D. A. P and Q are non-superimposable mirror images of each other. B. R and S are non-superimposable images of each other. C. Q and R are same compound. D. Q and S are enantiomers. E. statements A and B are correct.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



