
Concept explainers
(a)
Interpretation:
The oxidized product of the following alcohol when oxidized with
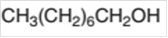
Concept Introduction:
A
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
The oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 14.69P
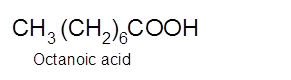
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or
carboxylic acid as it is overall removal of H atoms. - Primary alcohols are oxidized to
aldehyde which further oxidized to a carboxylic acid. - Secondary alcohols are oxidized to a
ketone (R2CO). - Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
Hence, the oxidization of 1-octanol will form octanoic acid molecule as 1-octanol is a primary alcohol.
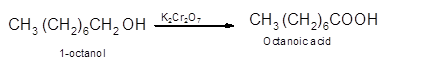
(b)
Interpretation:
The oxidized product of the following alcohol when oxidized with
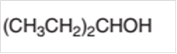
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
The oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 14.69P
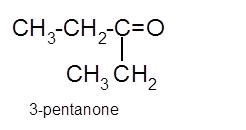
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or carboxylic acid as it is overall removal of H atoms.
- Primary alcohols are oxidized to aldehyde which further oxidized to a carboxylic acid.
- Secondary alcohols are oxidized to a ketone (R2CO).
- Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
Hence the oxidization of 3-pentanol will form 3-pentanone molecule as 3-pentanol is a secondary alcohol.
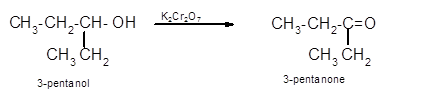
(c)
Interpretation:
The oxidized product of the following alcohol when oxidized with
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
Oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 14.69P
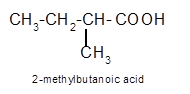
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or carboxylic acid as it is overall removal of H atoms.
- Primary alcohols are oxidized to aldehyde which further oxidized to carboxylic acid.
- Secondary alcohols are oxidized to ketone (R2CO).
- Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
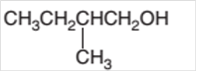
Hence the oxidization of 3-pentanol will form 3-pentanone molecule as 3-pentanol is a secondary alcohol.
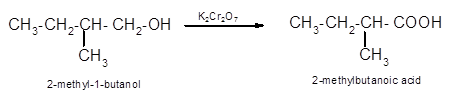
(d)
Interpretation:
The oxidized product of following alcohol when oxidized with
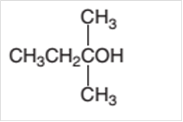
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactant and products must be separated by an arrow.
Oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 14.69P
2-ethyl-2-propanol cannot oxidize as it is a tertiary alcohol.
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or carboxylic acid as it is the overall removal of H atoms.
- Primary alcohols are oxidized to aldehyde which further oxidized to the carboxylic acid.
- Secondary alcohol is oxidized to ketone (R2CO).
- Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
Hence 2-ethyl-2-propanol cannot oxidize as it is a tertiary alcohol.
Want to see more full solutions like this?
Chapter 14 Solutions
General, Organic, & Biological Chemistry
- Draw the eight constitutional isomers with molecular formula C5H120 that contain an OH group. Give the IUPAC name for each compound. Classify each alcohol as 1°, 2°, or 3°.arrow_forwardWhat products are formed when an alcohol undergoes dehydration?arrow_forward1) Draw each alcohol2) Categorize the alcohol as primary, secondary or tertiary3) Oxidize each alcohol as many times as possible4) Name the product(s) if there are any molecule: 3-Tertbutylcyclopropanolarrow_forward
- An alcohol does not react with aziridine unless an acid is present. Why is the acid necessary?arrow_forward7) Oxidation of a 1° alcohol with chromic acid results in the production of A) an ester D) an acid B) a ketone C) an aldehyde E) none of the abovearrow_forwardOrganic Reaction Write an equation for the oxidation of each alcohol. Use [O] above the arrow to indicate an oxidizing agent. If no reaction occurs, write "no reaction" after the arrow. 1. CH3CH2CH2CH2CH2OH CH₂ CH₂CCH₂CH₂ ОН CH CHCH CH CH CH, ОНarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




