
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 52P
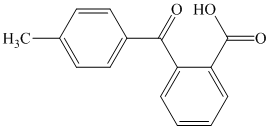
What combination of acyl chloride or acid anhydride and arene would you choose to
prepare each of the following compounds by Friedel–Crafts acylation?
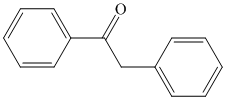
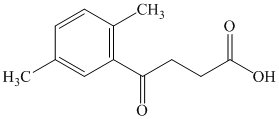
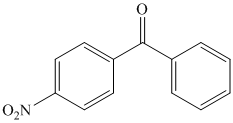
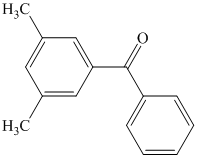
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain how the class I carbonyl compound reacts?
What will be the product when ethylamine and propyl amine reacts with acetyl chloride?
Why only one amide obtained after the reaction of acetyl chloride with a mixture of ethylamine and trimethylamine?
Excess amine is required in the reaction of acetyl chloride with amine whereas excess alcohol is not required in the reaction of acetyl chloride and alcohol. Why?
List the following ester in order of decreasing reactivities towards hydrolysis with reason:
Methyl benzoate, p-nitro methyl benzoate and p-methoxy methyl benzoate
Linalool and lavandulol are two of the major components of lavender oil. (a) What organolithium reagent and carbonyl compound can be used to make each alcohol? (b) How might lavandulol be formed by reduction of a carbonyl compound? (c) Why can't linalool be prepared by a similar pathway?
arrange the following compounds in order of decreasing reactivity towards hydrolysis
Chapter 13 Solutions
Organic Chemistry - Standalone book
Ch. 13.2 - Based on Hammonds postulate which holds that the...Ch. 13.3 - Prob. 2PCh. 13.3 - Using : O =N+= O : as the electrophile, write a...Ch. 13.4 - Prob. 4PCh. 13.5 - Prob. 5PCh. 13.6 - Prob. 6PCh. 13.6 - Write a reasonable mechanism for the formation of...Ch. 13.6 - tert-Butylbenzene can be prepared by alkylation of...Ch. 13.6 - Prob. 9PCh. 13.7 - The reaction shown gives a single product in 88...
Ch. 13.7 - Prob. 11PCh. 13.8 - Using benzene and any necessary organic or...Ch. 13.10 - Prob. 13PCh. 13.11 - Prob. 14PCh. 13.12 - Prob. 15PCh. 13.12 - Prob. 16PCh. 13.13 - Prob. 17PCh. 13.13 - Prob. 18PCh. 13.14 - Reaction of chlorobenzene with p-chlorobenzyl...Ch. 13.15 - Prob. 20PCh. 13.15 - Prob. 21PCh. 13.15 - Prob. 22PCh. 13.16 - Prob. 23PCh. 13.16 - Prob. 24PCh. 13.17 - Prob. 25PCh. 13.18 - Prob. 26PCh. 13.19 - Write the structure of the expected product from...Ch. 13.20 - Prob. 28PCh. 13.20 - Prob. 29PCh. 13.21 - Prob. 30PCh. 13.21 - Offer an explanation for the observation that...Ch. 13.21 - Prob. 32PCh. 13 - Write the structure of the organic product in each...Ch. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Prob. 36PCh. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Treatment of the alcohol shown with sulphuric acid...Ch. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - Arrange the following five compounds in order of...Ch. 13 - Prob. 45PCh. 13 - Prob. 46PCh. 13 - Prob. 47PCh. 13 - Give reagents suitable for carrying out each of...Ch. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Which is the best synthesis of the compound shown?Ch. 13 - What combination of acyl chloride or acid...Ch. 13 - A standard synthetic sequence for building a...Ch. 13 - Prob. 54PCh. 13 - Prob. 55PCh. 13 - Prob. 56PCh. 13 - Prob. 57PCh. 13 - Prob. 58PCh. 13 - Prob. 59PCh. 13 - Prob. 60DSPCh. 13 - Prob. 61DSPCh. 13 - Prob. 62DSPCh. 13 - Prob. 63DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Carbonyl compounds can be protonated on the carbonyl oxygen. Protonation of the carbonyl oxygen gives a species whose positive charge is delocalized by resonance. Explain why acetic acid (ethanoic acid) is more readily protonated than acetone (propanone).arrow_forwardTwo unsymmetrical anhydrides react with ethylamine as follows: Explain the factors that might account for the formation of the products in each reaction.arrow_forwardArrange the following carbonyl compounds in INCREASING REACTIVITY towards nucleophilic addition.arrow_forward
- Primary amines can also be prepared by the reaction of an alkyl halide with azide ion, followed by catalytic hydrogenation. What advantage do this method and the Gabriel synthesis have over the synthesis of a primaryamine using an alkyl halide and ammonia?arrow_forwardPrimary amines can also be prepared by the reaction of an alkyl halide with azide ion, followed by catalytic hydrogenation. What advantage do this method and the Gabriel synthesis have over the synthesis of a primary amine using an alkyl halide and ammonia?arrow_forwardWhat acid chloride would be needed to prepare each of the following ketones from benzene using a Friedel–Crafts acylation?arrow_forward
- Rank the following carboxylic acid derivatives in decreasing order (most to least) of reactivity towards nucleophilic acyl substitution.arrow_forwardName and Draw the structures of all possible chemical (Electrophilic aromatic substitution) reactions of the Phenol compound namely; Nitration Sulphonationarrow_forwardArrange the following compounds in order of increasing reactivity towards electrophilic aromatic substitution: Aniline Benzoic acid Chlorobenzene Ethylbenzenearrow_forward
- Suggest a reasonable synthesis for the following compounds from benzene.arrow_forwardArrange the the compounds in order of increasing reactivity towards electrophilic aromatic substitution.arrow_forwardProvide an explanation (in detail) on how base catalysts speed up nucleophilic reactions of carbonyl compounds.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License