
Concept explainers
(a)
Interpretation:
Whether hydration of 2-butene will give one or two products has to be identified based on Markovnikov’s rule.
Concept Introduction:
In this reaction no atoms or group of atoms are removed. Instead the unsaturated bond is reduced to saturated bond. A general scheme for addition reaction of
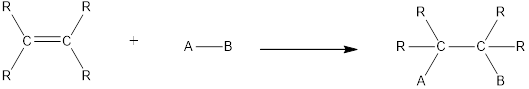
Addition reactions can be classified broadly into two types. They are asymmetrical addition reaction and symmetrical addition reaction.
Symmetrical addition reactions is the one in which the same atom or same group of atoms are added across the carbon‑carbon multiple bonds.
Unsymmetrical addition reactions is the one in which the different atom or different group of atoms are added across the carbon‑carbon multiple bonds.
Markovnikov’s rule:
When an unsymmetrical molecule of formula HQ to an unsymmeterical alkene, the hydrogen atom from HQ gets attached to the unsaturated carbon atom which has the most hydrogen atoms. In other words, it can be said that the hydrogen atom gets attached to the unsaturated carbon atom that is least substituted.
(b)
Interpretation:
Whether hydration of 2-pentene will give one or two products has to be identified based on Markovnikov’s rule.
Concept Introduction:
Chemical reaction in which an atom or a group of atoms are added to each carbon atom of a carbon‑carbon multiple bond in a hydrocarbon or hydrocarbon derivative is known as addition reaction.
In this reaction no atoms or group of atoms are removed. Instead the unsaturated bond is reduced to saturated bond. A general scheme for addition reaction of alkene can be given as shown below,

Addition reactions can be classified broadly into two types. They are asymmetrical addition reaction and symmetrical addition reaction.
Symmetrical addition reactions is the one in which the same atom or same group of atoms are added across the carbon‑carbon multiple bonds.
Unsymmetrical addition reactions is the one in which the different atom or different group of atoms are added across the carbon‑carbon multiple bonds.
Markovnikov’s rule:
When an unsymmetrical molecule of formula HQ to an unsymmeterical alkene, the hydrogen atom from HQ gets attached to the unsaturated carbon atom which has the most hydrogen atoms. In other words, it can be said that the hydrogen atom gets attached to the unsaturated carbon atom that is least substituted.
(c)
Interpretation:
Whether hydration of cyclobutene will give one or two products has to be identified based on Markovnikov’s rule.
Concept Introduction:
Chemical reaction in which an atom or a group of atoms are added to each carbon atom of a carbon‑carbon multiple bond in a hydrocarbon or hydrocarbon derivative is known as addition reaction.
In this reaction no atoms or group of atoms are removed. Instead the unsaturated bond is reduced to saturated bond. A general scheme for addition reaction of alkene can be given as shown below,

Addition reactions can be classified broadly into two types. They are asymmetrical addition reaction and symmetrical addition reaction.
Symmetrical addition reactions is the one in which the same atom or same group of atoms are added across the carbon‑carbon multiple bonds.
Unsymmetrical addition reactions is the one in which the different atom or different group of atoms are added across the carbon‑carbon multiple bonds.
Markovnikov’s rule:
When an unsymmetrical molecule of formula HQ to an unsymmeterical alkene, the hydrogen atom from HQ gets attached to the unsaturated carbon atom which has the most hydrogen atoms. In other words, it can be said that the hydrogen atom gets attached to the unsaturated carbon atom that is least substituted.
(d)
Interpretation:
Whether hydration of cyclohexene will give one or two products has to be identified based on Markovnikov’s rule.
Concept Introduction:
Chemical reaction in which an atom or a group of atoms are added to each carbon atom of a carbon‑carbon multiple bond in a hydrocarbon or hydrocarbon derivative is known as addition reaction.
In this reaction no atoms or group of atoms are removed. Instead the unsaturated bond is reduced to saturated bond. A general scheme for addition reaction of alkene can be given as shown below,

Addition reactions can be classified broadly into two types. They are asymmetrical addition reaction and symmetrical addition reaction.
Symmetrical addition reactions is the one in which the same atom or same group of atoms are added across the carbon‑carbon multiple bonds.
Unsymmetrical addition reactions is the one in which the different atom or different group of atoms are added across the carbon‑carbon multiple bonds.
Markovnikov’s rule:
When an unsymmetrical molecule of formula HQ to an unsymmeterical alkene, the hydrogen atom from HQ gets attached to the unsaturated carbon atom which has the most hydrogen atoms. In other words, it can be said that the hydrogen atom gets attached to the unsaturated carbon atom that is least substituted.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
General, Organic, and Biological Chemistry
- Alcohols are very useful starting materials for the production of many different compounds. The following conversions, starting with 1-butanol, can be carried out in two or more steps. Show the steps (reactants/catalysts) you would follow to carry out the conversions, drawing the formula for the organic product in each step. For each step, a major product must be produced. (See Exercise 62.) (Hint: In the presence of H+, an alcohol is converted into an alkene and water. This is the exact reverse of the reaction of adding water to an alkene to form an alcohol.) a. 1-butanol butane b. 1-butanol 2-butanonearrow_forward1. Using the grignard reaction of alkanes what is the resulting alkane if the reactant is C4H9Br? a. ethane b. propane c. butane d. pentane 2. Using the grignard reaction of alkanes what is the resulting alkane if the reactant is C5H11F? a. ethane b. propane c. butane d. pentane 3. Using Cl2 in C2H4Cl2 will result in HCl and ______. a. C2H3Cl3 b. C2H4Cl3 c. C2H2Cl3 d. not posiblearrow_forwardD. Ethene 73. The reagent KMN04 is used to completely oxidize acetylene. What is an expected property of the last oxidized form of acetylene? A. It will be soluble in hexane. B. It will exhibit acidic properties. C. It will form precipitate with NaCl. D. It will ionize into carbon and hydrogen. 74. As the molecular weight of alkenes increases, the boiling points also increase. Which of the following factors is best associated to this trend? A. Geometric isomerism B. Surface area C. Dipole interaction D. Structural isomerism 75. Which among the following compounds has the most electronegative carbon in its structure? A. Butyne B. Benzene C. Cyclobutane D. Benzaldehyde 76. Identify the SYSTEMATIC name of the molecule that is illustrated in this item, A. 2,4,6-trinitrotoluene B. 2-methyl-1,3,5-nitrobenzene C. 1-methyl-1,3,5-trinitrobenzene D. 1,3,5-trinitrotoluene CH3 NO2 O2N NO2 77 Identify the SYSTEMATIC name of the molecule that is illustrated in this item. A 1-methylbenzenesulfonic…arrow_forward
- Show how to convert 1- Butene into these compounds. a. Butane b. 2- Butanol c. 2- Bromobutane d. 1,2- Dibromobutanearrow_forwardName one structural isomer created by changing theposition of one or more halogen atoms in each alkylhalide.a. 2-chloropentane c. 1,3-dibromocyclopentaneb. 1,1-difluropropane d. 1-bromo-2-chloroethanearrow_forward1. Which of the following fuel has the lowest heat of combustion?a.Methaneb.Octanec.Coald.Ethanol 2. What is the process of breaking larger hydrocarbon molecules into smaller ones at low temperature through the use of catalyst in order to obtain higher quality of gasoline?a.Thermal crackingb.Catalytic crackingc.Catalytic reformingd.Catalytic combination 3. What is the most common oxidizing agent available in the atmosphere that can be used to generate fire?a.hydrogen peroxideb.oxygenc.ozoned.nitrous oxidearrow_forward
- Draw the structural formulas for each of the following organic compounds. Circle the non- alkyl functional groups a. hexan-2-one b. 2- methylpentanal c. Pentane-1,3-diol d. buta-1,3-diene e. 1-propoxybutane f. ethyl ethanoatearrow_forwardWhich of the following alcohols can be oxidized to a carboxylic acid? Name the carboxylic acid produced. Forthose alcohols that cannot be oxidized to a carboxylic acid,name the final product.a. Ethanol c. 1-Propanolb. 2-Propanol d. 3-Pentanolarrow_forwardThe product of the addition of HCl to an alkene is called a(n) a. alkane b. alkyne c. alkyl halide d. halo-alkenearrow_forward
- Give the reagent and the reaction conditions that would distinguish between the following compounds. Write the chemical reactions. a. benzene and cyclohexane b. 1-propene and 1-propyne c. ethyl benzene and acetylene d. 2-butene and butanearrow_forward1. What is the alkane product from the reaction of C7H13COONa and NaOH? a. pentane b. hexane c. butane d. heptane 2. What is the resulting alkane if we have C5H11F and a C5H11F as reactants in Wurtz synthesis? a. hexane b. octane c. nonane d. decane 3.What is the resulting alkane if we have 2C2H5Cl as reactants in Wurtz Synthesis reaction? a. ethane b. butane c. hexane d. octanearrow_forward9. The light initiated halogenation of alkanes involves the formation and reaction of which of the following intermediates? A. free radicals B. carbocations C. protonated halogens D. carbon anionsarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





