
Concept explainers
(a)
Interpretation:
The spatial arrangement for the
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and
Saturated hydrocarbons are
Alkane has general molecular formula as CnH2n+2. Alkene in which one double is present has general molecular formula as CnH2n. Alkyne in which one triple bond is present has the general molecular formula as CnH2n-2. Cycloalkanes have the general molecular formula as CnH2n. Cycloalkenes in which one double bond is present have the general molecular formula as CnH2n-2.
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(a)
Answer to Problem 13.37EP
The spatial arrangement is identified as tetrahedral.
Explanation of Solution
Given structure is,

Looking into the left most carbon atom present in the given structure, it is not bonded to any double bonds or triple bond. This carbon atom has only four single bonds (three with hydrogen and one with carbon atom). Therefore, the spatial arrangement of the left-most carbon atom is tetrahedral.
The spatial arrangement of the left-most carbon atom is identified.
(b)
Interpretation:
The spatial arrangement for the chemical bonds in the left‑most carbon atom in the given structure has to be identified.
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Saturated hydrocarbons are alkanes. Unsaturated hydrocarbons are alkene, alkyne and aromatic hydrocarbons.
Alkane has general molecular formula as CnH2n+2. Alkene in which one double is present has general molecular formula as CnH2n. Alkyne in which one triple bond is present has the general molecular formula as CnH2n-2. Cycloalkanes have the general molecular formula as CnH2n. Cycloalkenes in which one double bond is present have the general molecular formula as CnH2n-2.
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(b)
Answer to Problem 13.37EP
The spatial arrangement is identified as tetrahedral.
Explanation of Solution
Given structure is,

Looking into the left most carbon atom present in the given structure, it is not bonded to any double bonds or triple bond. This carbon atom has only four single bonds (three with hydrogen and one with carbon atom). Therefore, the spatial arrangement of the left-most carbon atom is tetrahedral.
The spatial arrangement of the left-most carbon atom is identified.
(c)
Interpretation:
The spatial arrangement for the chemical bonds in the left‑most carbon atom in the given structure has to be identified.
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Saturated hydrocarbons are alkanes. Unsaturated hydrocarbons are alkene, alkyne and aromatic hydrocarbons.
Alkane has general molecular formula as CnH2n+2. Alkene in which one double is present has general molecular formula as CnH2n. Alkyne in which one triple bond is present has the general molecular formula as CnH2n-2. Cycloalkanes have the general molecular formula as CnH2n. Cycloalkenes in which one double bond is present have the general molecular formula as CnH2n-2.
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(c)
Answer to Problem 13.37EP
The spatial arrangement is identified as trigonal planar.
Explanation of Solution
Given structure is,

Looking into the left most carbon atom present in the given structure, it is bonded to one double bond. This carbon atom has only two single bonds with hydrogen and a double bond with carbon atom. Therefore, the spatial arrangement of the left-most carbon atom is trigonal planar.
The spatial arrangement of the left-most carbon atom is identified.
(d)
Interpretation:
The spatial arrangement for the chemical bonds in the left‑most carbon atom in the given structure has to be identified.
Concept Introduction:
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Saturated hydrocarbons are alkanes. Unsaturated hydrocarbons are alkene, alkyne and aromatic hydrocarbons.
Alkane has general molecular formula as CnH2n+2. Alkene in which one double is present has general molecular formula as CnH2n. Alkyne in which one triple bond is present has the general molecular formula as CnH2n-2. Cycloalkanes have the general molecular formula as CnH2n. Cycloalkenes in which one double bond is present have the general molecular formula as CnH2n-2.
Considering the geometry of carbon atoms, the carbon atoms that have double bonds will have trigonal planar geometry. The carbon atoms that have only single bonds attached to it will have tetrahedral geometry. The carbon atoms that have a triple bond attached to it will have a linear geometry.
(d)
Answer to Problem 13.37EP
The spatial arrangement is identified as tetrahedral.
Explanation of Solution
Given structure is,
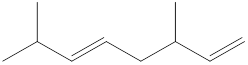
Looking into the left most carbon atom present in the given structure, it is not bonded to any double bonds or triple bond. This carbon atom has only four single bonds (three with hydrogen and one with carbon atom). Therefore, the spatial arrangement of the left-most carbon atom is tetrahedral.
The spatial arrangement of the left-most carbon atom is identified.
Want to see more full solutions like this?
Chapter 13 Solutions
General, Organic, and Biological Chemistry
- what is an intermittent water course and what kind of fish habitat it would providearrow_forwardwhy are native freshwater mussels are an important part of great lakes ecosystemarrow_forwardwhat morphological features differentiate the lamprey species and other species in the great lakesarrow_forward
- There are a wide range of therapeutic applications available as options for patients. Medical professionals should be aware of these applications so they can make informed recommendations to patients. To gain a better understanding of some therapeutic applications and how they are related to RNA and mRNA, research long non-coding RNA. Respond to the following in a minimum of 175 words: What is lncRNA and what does it do? How does IncRNA differ from mRNA? What are some therapeutic applications associated with lncRNA? Think about possible future uses of this application. What are the advantages and disadvantages of this application and its continued use?arrow_forwardfour fish or mussel species that are native to the great lakesarrow_forwardThere are a wide range of therapeutic applications available as options for patients. Medical professionals should be aware of these applications so they can make informed recommendations to patients. To gain a better understanding of some therapeutic applications and how they are related to RNA and mRNA, research long non-coding RNA. Respond to the following in a minimum of 175 words: What is lncRNA and what does it do? How does IncRNA differ from mRNA? What are some therapeutic applications associated with lncRNA? Think about possible future uses of this application. What are the advantages and disadvantages of this application and its continued use?arrow_forward
- four physial characteristics of a fish or a mussel that would help you identify it to a speciesarrow_forwarddescribe what you would do in this situation, you are working ona. river and it will take 20 minutes by boat to get back to the field truck, you are 1 hour from finishing the field work on the last day of field trip. you hear thunder int he dsitnace, what did you do?arrow_forwardunu grow because auxin is still produced in the tip to Another of Boysen and Jensen's experiments included the use of mica, explain why one of the shoots was able to show phototropism and the other was not. Mica Wafer Ligh c. They then t but this time permeable n shoot. Why phototropis Light Mica Wafer Coleoptile tips Tips removed: agar Explain why the shoo direction after the ag the cut shoot, even tarrow_forward
- Discussion entries must be at least 250 words to fulfill the assignment requirements. You must complete your entry before you will be able to see the responses of other students. Responses to other students are encouraged but not required. Grading for discussion entries will be based on application of course concepts, proper grammar, and correct punctuation. Read one the attached article and explore the Human Development Index (https://hdr.undp.org/data-center/human-development-index#/indicies/HDI). In your opinion, is the Human Development Index a good measure of the well- being of the people of a nation? Are the items measured in the HDI valid and relevant in the modern global economy? How are they related to the political economy of a nation? The attached articles propose some alternative measures of well-being. In your opinion, are there other measures of well-being that might be better alternatives to the items in the current HDI?arrow_forwardA patient visits her doctor with symptoms typical of a bladder infection. She is immediately prescribed an 800 mgdose of antibiotic (bioavailability = 1/2, t½ = 12 h). The corresponding plasma concentration of drug is found to be 96 micrograms/ml. What is the volume of distribution of this drug? Please round to the nearest liter.arrow_forwardA 10 mg/Kg dose of a drug is given by intravenous injection to a 20 Kg dog. What would the volume of distribution be if the drug had been given orally and only 50% of the drug was absorbed (the concentration of drug at time = 0 is 0.1 mg/L)? Be sure to show your work.arrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning





