
College Physics
2nd Edition
ISBN: 9780134601823
Author: ETKINA, Eugenia, Planinšič, G. (gorazd), Van Heuvelen, Alan
Publisher: Pearson,
expand_more
expand_more
format_list_bulleted
Question
Chapter 12, Problem 13MCQ
To determine
The graph which does not represent the process described by the equation
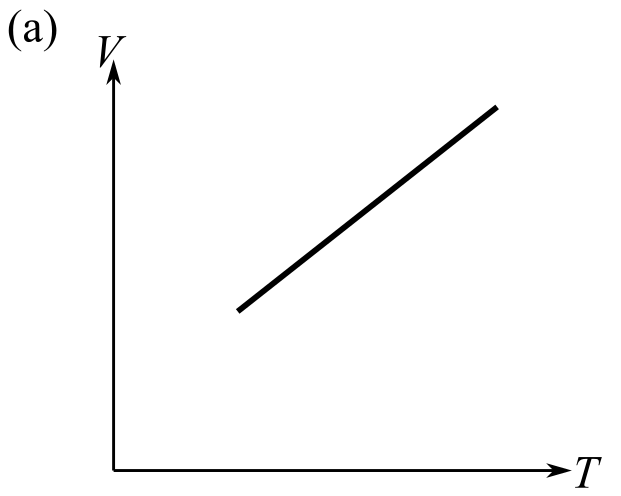
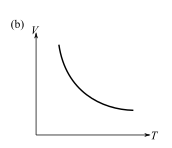
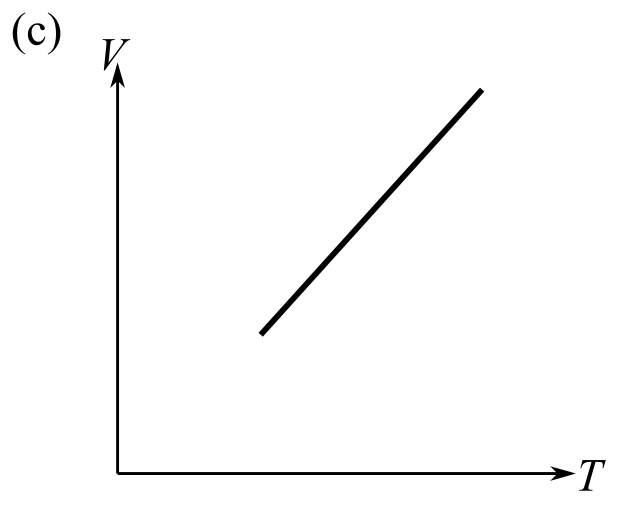
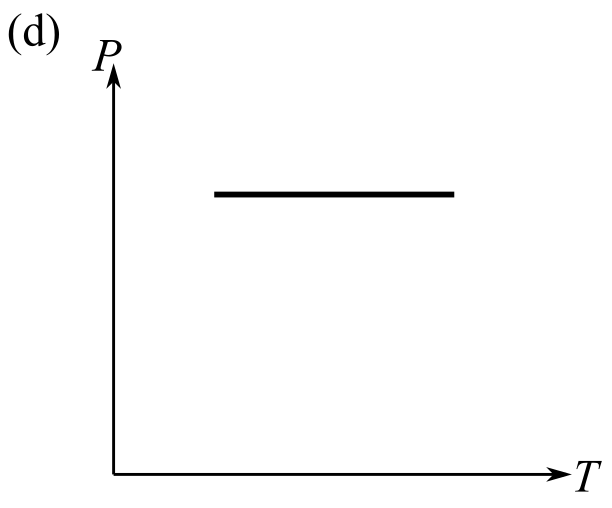
(e) a and b
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain why the relationship between pressure and volume of a gas at a set temperature and quantity is
an inverse variation. Answer in complete sentences.
UPLOAD
WRITER
C2
napter 14, Problem 14.1
Textbook Problem
1 views a
Prairie dogs ventilate their burrows by building a mound around one
entrance, which is open to a stream of air when wind Wows from any
direction. A second entrance at ground level is open to almost stagnant
air. How does this construction create an airflow through the burrow?
A pressure versus volume (pV) diagram for a system is shown
in the figure. The arrows of the curve indicate the direction of
the
process, and the points of interest are labeled. The values
for the points in the diagram are shown in the table.
Volume (m³)
Pressure (Pa)
Vo = 25.8
Po = 1.00 × 10ª
%3D
2
3
Vị
= 20.8
Рі 3D 1.00 х 104
V2 = 17.4
P2 = 5.34 × 103
V3
13.9
P3 =
5.34 x 103
4
V4 = 13.9
P4 = 3.20 × 10³
%3|
Vs = 9.55
P5 = 1.00 x 103
%3D
Calculate the amount of work done on the system from 0–2 (
Wo2) and then for the entire curve from 0-5 (Wo5).
Volume (m³)
79.81 x103
Wo2
J
Incorrect
107.09 x103
Wo5
J
Incorrect
Pressure (Pa)
Chapter 12 Solutions
College Physics
Ch. 12 - Prob. 1RQCh. 12 - Prob. 2RQCh. 12 - Prob. 3RQCh. 12 - Review Question 12.4 Ken says that the temperature...Ch. 12 - Review Question 12.5 What is the difference...Ch. 12 - Prob. 6RQCh. 12 - Prob. 7RQCh. 12 - Review Question 12.8 How do we know that the Sun’s...Ch. 12 - Prob. 1MCQCh. 12 - Prob. 2MCQ
Ch. 12 - Prob. 3MCQCh. 12 - Prob. 4MCQCh. 12 - Prob. 5MCQCh. 12 - Prob. 6MCQCh. 12 - Prob. 7MCQCh. 12 - Prob. 8MCQCh. 12 - 9. How might physicists have come to know that at...Ch. 12 - 10. A cylindrical container is filled with a gas....Ch. 12 - Prob. 11MCQCh. 12 - A completely closed rigid container of gas is...Ch. 12 - Prob. 13MCQCh. 12 - Prob. 14MCQCh. 12 - Prob. 15MCQCh. 12 - Which of the following conditions are crucial for...Ch. 12 - Prob. 17CQCh. 12 - 18. Why does it hurt to walk barefoot on gravel?
Ch. 12 - 19. In the magic trick in which a person lies on a...Ch. 12 - What does it mean if the density of a gas is 1.29...Ch. 12 - How many oranges would you have if you had two...Ch. 12 - 22. Imagine that you have an unknown gas. What...Ch. 12 - Prob. 23CQCh. 12 - Describe how temperature and one degree are...Ch. 12 - Why does sugar dissolve faster in hot tea than in...Ch. 12 - 26. (a) Describe experiments that were used to...Ch. 12 - Give three examples of diffusion that are...Ch. 12 - Why do very light gases such as hydrogen not exist...Ch. 12 - Prob. 29CQCh. 12 - Explain why Earth has almost no free hydrogen in...Ch. 12 - What are the molar masses of molecular and atomic...Ch. 12 - Prob. 2PCh. 12 - The average particle density in the Milky Way...Ch. 12 - * (a) What is the concentration (number per cubic...Ch. 12 - Prob. 5PCh. 12 - 6. You find that the average gauge pressure in...Ch. 12 - Prob. 7PCh. 12 - Prob. 8PCh. 12 - Prob. 9PCh. 12 - 10. You have five molecules with the following...Ch. 12 - 11.Two gases in different containers have the same...Ch. 12 - 12. Four molecules are moving with the following...Ch. 12 - m2, what is the average pressure of the 10 tennis...Ch. 12 - * Friends throw snowballs at the wall of a...Ch. 12 - Prob. 15PCh. 12 - Prob. 16PCh. 12 - Prob. 17PCh. 12 - Air consists of many different molecules, for...Ch. 12 - Prob. 19PCh. 12 - 20. Air is a mixture of molecules of different...Ch. 12 - Prob. 21PCh. 12 - Prob. 22PCh. 12 - 23. ** A molecule moving at speed collides...Ch. 12 - Prob. 24PCh. 12 - Prob. 25PCh. 12 - * Even the best vacuum pumps cannot lower the...Ch. 12 - Prob. 27PCh. 12 - Prob. 28PCh. 12 - * The following data were collected for the...Ch. 12 - Prob. 30PCh. 12 - Prob. 31PCh. 12 - 32. * When surrounded by air at a pressure of 1.0...Ch. 12 - 33. * Some students are given the following...Ch. 12 - 34. ** You have gas in a container with a movable...Ch. 12 - Prob. 35PCh. 12 - * Bubbles While snorkeling, you see air bubbles...Ch. 12 - Prob. 37PCh. 12 - * Mount Everest (a) Determine the number of...Ch. 12 - Prob. 39PCh. 12 - Prob. 40PCh. 12 - Prob. 41PCh. 12 - 42. * Car tire dilemma Imagine a car tire that...Ch. 12 - 43. * There is a limit to how much gas can pass...Ch. 12 - Prob. 44PCh. 12 - Prob. 45PCh. 12 - 46. * In the morning, the gauge pressure in your...Ch. 12 - ** The P-versus-T graph in Figure P12.49 describes...Ch. 12 - ** The V-versus-T graph in Figure P12.50 describes...Ch. 12 - Prob. 51PCh. 12 - Prob. 52PCh. 12 - Prob. 53PCh. 12 - 55. ** A gas that can be described by the ideal...Ch. 12 - * Equation Jeopardy 3 The three equations below...Ch. 12 - Prob. 57GPCh. 12 - 58. * See the previous problem Explain how the...Ch. 12 - Prob. 59GPCh. 12 - Prob. 60GPCh. 12 - Prob. 61GPCh. 12 - Prob. 62GPCh. 12 - 63. EST * Car engine During a compression stroke...Ch. 12 - * How can the pressure of air in your house stay...Ch. 12 - 65 * Tell-all problem Tell everything you can...Ch. 12 - 66. ** Two massless, frictionless pistons are...Ch. 12 - 67. * A closed cylindrical container is divided...Ch. 12 - Prob. 68GPCh. 12 - 69. ** The speed of sound in an ideal gas is given...Ch. 12 - 70. * Using the information from problem 12.69,...Ch. 12 - Prob. 71GPCh. 12 - 73. Why is the wall tension in capillaries so...Ch. 12 - Prob. 74RPPCh. 12 - Prob. 75RPPCh. 12 - As a person ages, the fibers in arteries become...Ch. 12 - Prob. 77RPPCh. 12 - The bag and pump have a 6.76-kg mass. The volume...Ch. 12 - The bag and pump have a 6.76-kg mass. The volume...Ch. 12 - The bag and pump have a 6.76-kg mass. The volume...Ch. 12 - The bag and pump have a 6.76-kg mass. The volume...Ch. 12 - Prob. 82RPP
Knowledge Booster
Similar questions
- A gas is in a container of volume V0 at pressure P0. It is being pumped out of the container by a piston pump. Each stroke of the piston removes a volume Vs through valve A and then pushes the air out through valve B as shown in Figure P19.74. Derive an expression that relates the pressure Pn of the remaining gas to the number of strokes n that have been applied to the container. FIGURE P19.74arrow_forward(a) Show that the density of an ideal gas occupying a volume V is given by = PM/KT, where M is the molar mass. (b) Determine the density of oxygen gas at atmospheric pressure and 20.0C.arrow_forward(a) Use the ideal gas equation to estimate the temperature at which 1.00 kg of steam (molar mass M=18.0 g/mol) at a pressure of 1.50106 Pa occupies a volume of 0.220 m3. (b) The van der Waals constants for water are a=0.5537 Pa m6/mol2 and b=3.049105 m3/mol. Use the Van der Waals equation of state to estimate the temperature under the same conditions. (c) The actual temperature is 779 K. Which estimate is better? `arrow_forward
- Engineers are frequently called on to inspect and, if necessary, repair equipment in nuclear power plants. Suppose that the city lights go out. After inspecting the nuclear reactor, you find a leaky pipe that leads from the steam generator to turbine chamber, (a) How do the pressure readings for the turbine chamber and steam condenser compare? (b) Why is the nuclear reactor not generating electricity?arrow_forwardA vertical cylinder of cross-sectional area A is fitted with a tight-fitting, frictionless piston of mass m (Fig. P18.40). The piston is not restricted in its motion in any way and is supported by the gas at pressure P below it. Atmospheric pressure is P0. We wish to find the height h in Figure P18.40. (a) What analysis model is appropriate to describe the piston? (b) Write an appropriate force equation for the piston from this analysis model in terms of P, P0, m, A, and g. (c) Suppose n moles of an ideal gas are in the cylinder at a temperature of T. Substitute for P in your answer to part (b) to find the height h of the piston above the bottom of the cylinder. Figure P18.40arrow_forwardA sealed cubical container 20.0 cm on a side contains a gas with three times Avogadros number of neon atoms at a temperature of 20.0C. (a) Find the internal energy of the gas. (b) Find the total translational kinetic energy of the gas. (c) Calculate the average kinetic energy per atom, (d) Use Equation 10.13 to calculate the gas pressure. (e) Calculate the gas pressure using the ideal gas law (Eq. 10.8).arrow_forward
- A deepsea diver should breathe a gas mixture that has the same oxygen partial pressure as at sea level, where dry air contains 20.9% oxygen and has a total pressure of 1.01105N/m2. (a) What is me partial pressure of oxygen at sea level? (b) If the diver breathes a gas mixture at a pressure of 2.00106N/m2, what percent oxygen should it be to have the same oxygen partial pressure as at sea level?arrow_forwardRefer to Problem 16 and Figure P14.16. A hydrometer is to be constructed with a cylindrical floating rod. Nine fiduciary marks are to be placed along the rod to indicate densities of 0.98 g/cm3, 1.00 g/cm3, 1.02 g/cm3, 1.01 g/cm3, 1.14 g/cm3. The row of marks is to start 0.200 cm from the top end of the rod and end 1.80 cm from the top end. (a) What is the required length of the rod? (b) What must be its average density? (c) Should the marks be equally spaced? Explain your answer.arrow_forwardThe average human has a density of 945 kg/m3 after in haling and 1 020 kg/m3 after exhaling. (a) Without making any swimming movements, what percentage of the human body would be above the surface in the Dead Sea (a body of water with a density of about 1 230 kg/m3) in each of these cases? (b) Given that bone and muscle are denser than fat, what physical characteristics differentiate sinkers (those who tend to sink in water) from floaters (those who readily float)?arrow_forward
- Review. (a) Derive an expression for the buoyant force on a spherical balloon, submerged in water, as a function of the depth h below the surface, the volume Vi of the balloon at the surface, the pressure P0 at the surface, and the density w of the water. Assume the water temperature does not change with depth, (b) Does the bouyant force increase or decrease as the balloon is submerged? (c) At what depth is the buoyant force one-half the surface value?arrow_forwardA pressure versus volume (pv) diagram for a system is shown in the figure. The arrows of the curve indicate the direction of the process, and the points of interest are labeled. The values for the points in the diagram are shown in the table. Volume (m3) Pressure (Pa) v0=27.4 p0=1.00×104 v1=19.3 p1=1.00×104 v2=16.0 p2=4.92×103 v3=13.3 p3=4.92×103 v4=13.3 p4=3.20×103 v5=7.51 p5=1.00×103 Calculate the amount of work done on the system from 0–2 (W02) and then for the entire curve from 0–5 (W05).arrow_forwardA food coloring has a diffusion constant in water of 10 x 10–10 m2/s. A difference of food coloring concentration of 20 g/m3 is maintained between the ends of a cylinder with a cross-sectional area of 5 cm2 and a length of 8 cm. How much mass of food coloring diffuses though the cylinder in 20 minutes? A.1.5 x 10-10 kg B.1.5 x 10-8 kg C.1.5 x 10-6 kg D.1.5 x 10-4 kg E.1.5 x 10-2 kgarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax