
Concept explainers
(a)
Interpretation:
The molecular formula for the given cycloalkane structure has to be found.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and
Cycloalkanes are a class of saturated hydrocarbons that contain a ring of carbon atoms with or without alkyl substituents on it. The general molecular formula for cycloalkanes is
In a line-angle structural formula of cycloalkanes, the intersection of two lines represent a methylene group (
(a)
Answer to Problem 12.96EP
The molecular formula for the given cycloalkane is
Explanation of Solution
Given cycloalkane is,

The above structure is a line-angle structural formula representation. This structure has a total of five intersections. Therefore, the total number of carbon atoms present in the given structure is found to be five.
The molecular formula for the given cycloalkane can be found using the general molecular formula. General molecular formula for cycloalkane is
The molecular formula for the given cycloalkane is found to be
The molecular formula for the given cycloalkane is found out using the general molecular formula for cycloalkanes.
(b)
Interpretation:
The molecular formula for the given cycloalkane structure has to be found.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Cycloalkanes are a class of saturated hydrocarbons that contain a ring of carbon atoms with or without alkyl substituents on it. The general molecular formula for cycloalkanes is
In a line-angle structural formula of cycloalkanes, the intersection of two lines represent a methylene group (
(b)
Answer to Problem 12.96EP
The molecular formula for the given cycloalkane is
Explanation of Solution
Given cycloalkane is,
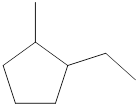
The above structure is a line-angle structural formula representation. This structure has a total of six intersections and two end lines. Therefore, the total number of carbon atoms present in the given structure is found to be eight.
The molecular formula for the given cycloalkane can be found using the general molecular formula. General molecular formula for cycloalkane is
The molecular formula for the given cycloalkane is found to be
The molecular formula for the given cycloalkane is found out using the general molecular formula for cycloalkanes.
(c)
Interpretation:
The molecular formula for the given cycloalkane structure has to be found.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Cycloalkanes are a class of saturated hydrocarbons that contain a ring of carbon atoms with or without alkyl substituents on it. The general molecular formula for cycloalkanes is
In a line-angle structural formula of cycloalkanes, the intersection of two lines represent a methylene group (
(c)
Answer to Problem 12.96EP
The molecular formula for the given cycloalkane is
Explanation of Solution
Given cycloalkane is,
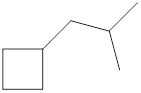
The above structure is a line-angle structural formula representation. This structure has a total of six intersections and two end lines. Therefore, the total number of carbon atoms present in the given structure is found to be eight.
The molecular formula for the given cycloalkane can be found using the general molecular formula. General molecular formula for cycloalkane is
The molecular formula for the given cycloalkane is found to be
The molecular formula for the given cycloalkane is found out using the general molecular formula for cycloalkanes.
(d)
Interpretation:
The molecular formula for the given cycloalkane structure has to be found.
Concept Introduction:
Organic compounds are the important basis of life. They include gasoline, coal, dyes, and clothing fibers etc. The compounds that are obtained from living organisms are termed as organic compounds and those obtained from the earth are known as inorganic compounds. Organic compounds are found in earth also apart from living organisms. All the organic compounds contain the element carbon. Urea was synthesized in the laboratory which is an organic compound.
Hydrocarbons are the organic compounds that contain only hydrogen and carbon atoms. Hydrocarbon derivatives are the one in which the compounds contain hydrogen and carbon atoms along with one or more additional elements. The additional elements that can be present in hydrocarbon derivatives are oxygen, nitrogen, sulphur, chlorine, bromine etc.
Hydrocarbons are further classified into two categories. They are saturated hydrocarbons and unsaturated hydrocarbons. The hydrocarbons that contain single bonds between carbon atoms in the entire molecule is known as saturated hydrocarbon. The hydrocarbons that contain atleast one double or triple bond between two carbon atoms in the entire molecule is known as unsaturated hydrocarbon.
Cycloalkanes are a class of saturated hydrocarbons that contain a ring of carbon atoms with or without alkyl substituents on it. The general molecular formula for cycloalkanes is
In a line-angle structural formula of cycloalkanes, the intersection of two lines represent a methylene group (
(d)
Answer to Problem 12.96EP
The molecular formula for the given cycloalkane is
Explanation of Solution
Given cycloalkane is,
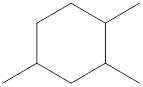
The above structure is a line-angle structural formula representation. This structure has a total of six intersections and three end lines. Therefore, the total number of carbon atoms present in the given structure is found to be nine.
The molecular formula for the given cycloalkane can be found using the general molecular formula. General molecular formula for cycloalkane is
The molecular formula for the given cycloalkane is found to be
The molecular formula for the given cycloalkane is found out using the general molecular formula for cycloalkanes.
Want to see more full solutions like this?
Chapter 12 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forward
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardOnly 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve itarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





