
Concept explainers
Chemical/Bio Engineering
An irreversible, first-order reaction takes place in four well mixed reactors (Fig. P12.10),
Thus, the rate at which A is transformed to B can be represented as
The reactors have different volumes, and because they are operated at different temperatures, each has a different reaction rate:
| Reactor | V, L |
|
| 1 | 25 | 0.05 |
| 2 | 75 | 0.1 |
| 3 | 100 | 0.5 |
| 4 | 25 | 0.1 |
Determine the concentration of A and B in each of the reactors at steady state.
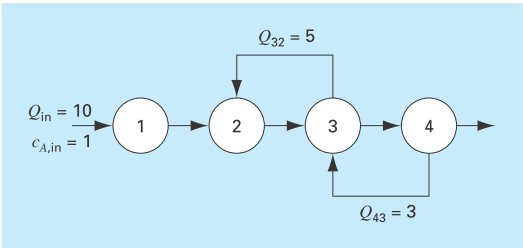
FIGURE P12.10
To calculate:
Answer to Problem 10P
Solution:
Explanation of Solution
Given Information:
Write the provided values of the volume and rate of reaction.
Formula used:
Write system of linear equations in matrix form.
And,
The term
Calculation:
Consider the provided diagram for an irreversible first order reaction takes place in four will-mixed reactors.
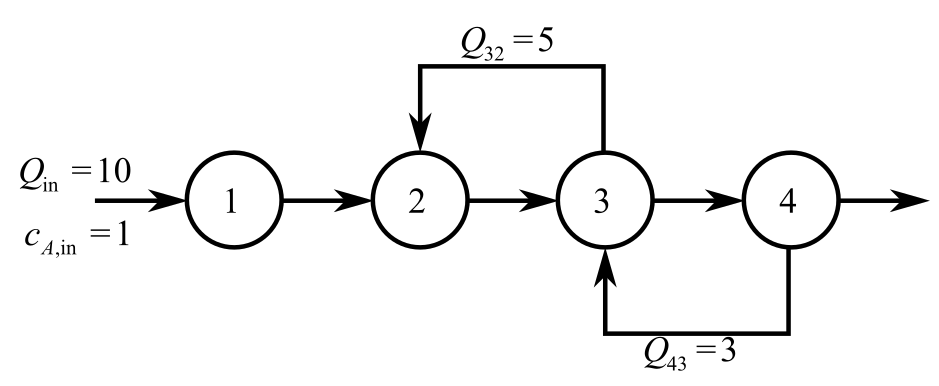
Balance the mass for A in reactor 1 at steady-state.
Substitute
Further solve.
Balance the mass for A in reactor 2 at steady-state.
Substitute
Further solve.
Balance the mass for A in reactor 3 at steady-state.
Substitute
Further solve.
Balance the mass for A in reactor 4 at steady-state.
Substitute
Further solve.
Balance the mass for B in reactor 1 at steady-state.
Substitute
Further solve.
Balance the mass for B in reactor 2 at steady-state.
Substitute
Further solve.
Balance the mass for B in reactor 3 at steady-state.
Substitute
Further solve.
Balance the mass for B in reactor 4 at steady-state.
Substitute
Further solve.
Now, write all the equations, to find linear system of equations.
Write the above linear equations in matrix form as written in symbolized form.
Here, coefficient matrix A is,
Column matrix
Column matrix B is,
Substitute the values in the matrix equation form.
Solve for
Code:
Type the above code into MATLAB command window and press enter to find the result.
Result is obtained as follows:
Hence,
Hence, the concentration of A and B is,
Want to see more full solutions like this?
Chapter 12 Solutions
EBK NUMERICAL METHODS FOR ENGINEERS
Additional Engineering Textbook Solutions
Advanced Engineering Mathematics
Basic Technical Mathematics
Fundamentals of Differential Equations (9th Edition)
Calculus: Single And Multivariable
A Problem Solving Approach To Mathematics For Elementary School Teachers (13th Edition)
- 3 A farmer has a 10-heactare maize farm. The maize is under “Fall Army Worm” attack. The farmer has to urgently control the attack using a chemical called AGENOX 221. Motorized sprayers with tank capacities of 15 litres each are to be used. The recommended concentration of the chemical is 2 ml/L and each full tank will cover ½ hectare. A 50-man team is engaged to control the attack. Using the above information and the assumptions given below: i. What volume of the chemical is required to cover the entire field to control the “Fall Army Worm” attack? ii. If each man covers an area of 25 m2 from one end of the field to the other, how many trips will each of them make to cover the entire field? Assume ach man moves in a straight line. Assumptions: 1. The 10 ha farm is considered to be a perfect square. 2. There is a 2.5 % loss of chemical due to the transfer to the sprayer tank.arrow_forward10:45 PM | 5.7KB/s E expert.chegg.com/ex Chegg + Time remaining: 00:06:48 Vo 4G+ LTE Answer Skip Exit 10 26 Chemistry 1. You are throwing a fondue party! You need to heat 800.0 mL of vegetable oil from 20 to 190.0°C using an ethanol burner. Assuming a perfect transfer of heat from the burner into the oil, what mass of ethanol would be required (Note - ethanol has a molar enthalpy of -1277 KJ/mol)? Assume your kitchen is at SATP, vegetable oil has a density of 0.920 g/mL and the specific heat capacity of vegetable oil is 2.000 J/g °C.arrow_forward= 14 15 16 17 18 19 20 21 22 23 24 25 26 A gas storage cylinder in an ordinary chemical laboratory measures 3.9 cm wide and 16. cm high. This is the label on it. olo Contents: N, gas Pressure: 7.93 atm If the cylinder is opened and the gas allowed to escape into a large empty plastic bag, what will be the final volume of nitrogen gas, including what's collected in the plastic bag and what's left over in the cylinder? Write your answer in liters. Round your answer to 2 significant digits. ?arrow_forward
- BME2223-ENGINEERING MECHANICS MIDTERM E) 1) Find V.(V× B) for B = 3x²yî – 2xyzĵ + 5y²z³karrow_forwardA closed thermodynamic system consists of a fixed amount of substance (i.e. mass) in which no substance can flow across the boundary, but energy can. For a closed themodynamic system we cannot add energy to the system, via substance (E ) (1.e. matter which contains energy is not allowed across the boundary) Across the Boundaries E° = No Q = = Yes W mass NO CLOSED = Yes SY STEM m = constant | energy YES Figure 1.1. If the substance inside the thermodynamic system shown in figure 1.1. (i.e. piston cylinder device) is air, is the system a Fixed closed system Moveable closed system A. В.arrow_forwardthermodynamics Ten kilogram of water us mixed with 12 kilogram of alcohol (SG=0.9).What is the specific gravity of the mixture, assuming that two fluidsmix completely.arrow_forward
- 49.) When control volume is losing heat, the rate of heat transfer is negative (sign convention). Select one: True False 47.) The properties of the saturated liquid are the same whether it exists alone or in a mixture with saturated vapor. Select one: True Falsearrow_forward4. A nuclear power facility produces a vast amount of heat, which is usually discharged into aquatic systems. This heat raises the temperature of the aquatic system, resulting in a greater concentration of chlorophyll a, which in turn extends the growing season. To study this effect, water samples were collected monthly at 3 stations for a period of 12 months. Štation Á is located closest to a potential heated water discharge, station Č is located farthest away from the discharge, and station B is located halfway between stations A and C. The following concentrations of chlorophyll a were recorded. Station Month January February March A B C 9.867 3.723 8.416 4.410 14.035 11.100 10.700 20.723 4.470 April Мay June July August September October November December 13.853 9.168 8.010 7.067 4.778 34.080 11.670 9.145 8.990 7.357 8.463 3.350 3.358 4.086 4.500 4.210 4.233 6.830 3.630 2.320 5.800 2.953 3.843 3.480 2.640 3.610 3.020 Perform an analysis of variance and test the hypothesis, at the…arrow_forwardThe marketing research department of a large manufacturing company has determined that the demand equations for two major items it produces are given by p = 2,000 - 5x + 8y and q = 4,000+ 9x - 7y where p is the price of item A. is the price of item B , x is the monthly demand for item A , and y is the monthly demand for item B. Find the total monthly revenue from items A and B when x = 15 and y = 5 .arrow_forward
- 3. A tank contains 100 gal of brine in which 50 lb of salt is dissolved. Brine containing 2 lb/gal of salt runs into the tank at the rate of 3 gal/min, and the mixture, assumed to be kept uniform by stirring, runs out at 2 gal/min. How much salt is in the tank at the end of 30 min? 4. What is the equation of a family of curves orthogonal to 2r + y? = c? 5. A resistance R = 10 ohms and capacitance C = 4 millifarad are connected in series with a direct current element V = 50 volts. Assume that when the switch is closed, the charge on the capacitor is 0.02 coulomb. Find the initial current.arrow_forwardEquations of Polytropic Process Definition, diagrams, equations, & sample problems Test Your Skills 14.1 1. A substance undergoes a process such that pV13 = C. If during this process the volume increases by 25% and the initial temperature is 300 K, find the final temperature in K. Your answer Submit Previous activity Jumn to 合曲 60000arrow_forwardThe table below shows the melting point, specific heat, and heat of fusion of different solids. Suppose the same mass of these solids are at their respective melting point temperatures, which solid requires most amount of heat to melt? Substance Melting Point Heat of Fusion Specific Heat 79.71 cal/g 5.85 cal/g 21.07 cal/g 15.39 cal/g 31.98 cal/g 0.50 cal/gC° 0.033 cal/gC° 0.056 cal/gC° 0.030 cal/gCo 0.093 cal/gC° Ice 0°C Lead 327°C Silver 961°C Gold 1063°C Copper 1083°C O lead ice O gold O silver O O O Oarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





