
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11.9, Problem 13P
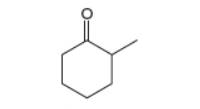
(a) Draw two different enol tautomers of
constitutional isomers that are not tautomers, but contain a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(a) Draw the structure of the hemiacetal formed from one mole of benzaldehyde and one mole of
ethanol.
(b) Draw the structure of the acetal formed from one mole of benzaldehyde and two moles of ethanol.
(c) Draw the structure of 2-methoxy-2-butanol. What compounds could you prepare this from?
(d) Draw the structure of 3-methoxyl-2-butanol. What functional groups are present? Is this an acetal, a
hemiacetal, or neither? Explain.
(e) Identify the functional groups in the molecules shown below. Circle any acetals or hemiacetal, and
identify which they are.
0-
(a) Draw the structure of the hemiacetal formed from one mole of benzaldehyde and one mole of
ethanol.
(b) Draw the structure of the acetal formed from one mole of benzaldehyde and two moles of ethanol.
(c) Draw the structure of 2-methoxy-2-butanol. What compounds could you prepare this from?
c)
rite in the reagent(s) over the arrow.
a) C6H5N₂+
b) C6H5C=N
H3C-
An
OH
H₂C
→ benzene
H3C
benzylamine
CI
CH3
Chapter 11 Solutions
Organic Chemistry (6th Edition)
Ch. 11.1 - Problem 11.1 Neopheliosyne B is a novel acetylenic...Ch. 11.2 - Give the IUPAC name for each compound.Ch. 11.2 - Give the structures corresponding to each of the...Ch. 11.3 - Prob. 4PCh. 11.5 - Prob. 5PCh. 11.6 - Which bases can deprotonate acetylene? The pKa...Ch. 11.7 - Draw the organic products formed when each alkyne...Ch. 11.7 - Draw additional resonance structures for each...Ch. 11.8 - Problem 11.9 Draw the products formed when is...Ch. 11.8 - Explain the following result. Although alkenes...
Ch. 11.9 - Problem 11.11 Draw the keto tautomer of each...Ch. 11.9 - Prob. 12PCh. 11.9 - a Draw two different enol tautomers of...Ch. 11.10 - Prob. 14PCh. 11.10 - Problem 11.15 Draw the organic products formed in...Ch. 11.11 - Problem 11.16 What acetylide anion and alkyl...Ch. 11.11 - Problem. 11.17 Show how , and can be used to...Ch. 11.11 - Prob. 18PCh. 11.11 - Draw the products of each reaction. a. b.Ch. 11.11 - Prob. 20PCh. 11 - Prob. 25PCh. 11 - 11.25 Answer the following questions about...Ch. 11 - 11.26 Give the IUPAC name for each alkyne.
a. ...Ch. 11 - Prob. 28PCh. 11 - Which of the following pairs of compounds...Ch. 11 - Prob. 30PCh. 11 - 11.30 How is each compound related to A? Choose...Ch. 11 - Prob. 32PCh. 11 - 11.33 Draw the products formed when is treated...Ch. 11 - What reagents are needed to convert (CH3CH2)3CCCH...Ch. 11 - 11.36 What alkynes give each of the following...Ch. 11 - 11.37 What alkyne gives each compound as the only...Ch. 11 - 11.38 Draw the organic products formed in each...Ch. 11 - 11.42 What reactions are needed to convert alcohol...Ch. 11 - 11.50 What acetylide anion and alkyl halide are...Ch. 11 - 11.52 Devise a synthesis of each compound using ...Ch. 11 - Prob. 58PCh. 11 - 11.59 N-Chlorosuccinimide (NCS) serves as a source...Ch. 11 - 11.60 Draw a stepwise mechanism for the following...Ch. 11 - 11.61 Draw a stepwise mechanism for the following...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe the orbitals used in bonding and the bond angles in the following compounds: a. CH3O b. CO2 c. H2CO d....
Organic Chemistry (8th Edition)
The chapter sections to review are shown in parentheses at the end of each problem. A "chemical-free” shampoo i...
Basic Chemistry
What is the pH range for acidic solutions? For basic solutions?
EBK INTRODUCTION TO CHEMISTRY
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Write the electron configurations far each of the following elements: (a) Sc. (b) Ti. (c) Cr. (d) Fe. (e) Ru
Chemistry by OpenStax (2015-05-04)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Dexamethasone is a halogen-containing steroid used to treat infl ammation in rheumatoid arthritis and other conditions. (a) Classify the alkyl halide in dexamethasone as 1 °, 2 °, or 3 °. (b) Classify the hydroxyl groups as 1 °, 2 °, or 3 °.arrow_forwardPredict which member of each group is most soluble in water, and explain the reasons for your predictions.(a) butan-1-ol, pentan-1-ol, or propan-2-ol(b) chlorocyclohexane, cyclohexanol, or cyclohexane-1,2-diol(c) phenol, cyclohexanol, or 4-methylcyclohexanolarrow_forward4 But-2-enal, CH₂CH=CHCHO, is a pale yellow, flammable liquid with an irritating odour. (a) But-2-enal exists as two stereoisomers. Draw skeletal formulae to show the structure of the two stereoisomers of but-2-enal. (b) (i) Describe a simple chemical test that would show that but-2-enal is an aldehyde. (ii) Explain why this test gives a different result with aldehydes than it does with keton (c) But-2-enal also reacts with sodium borohydride, NaBH4. (i) Identify the organic compound formed in this reaction. (ii) State the type of chemical reaction occurring. (d) Precautions must be taken to prevent but-2-enal catching fire. Construct a balanced equation for the complete combustion of but-2-enal, C₂HO.arrow_forward
- Draw the following compound.(2E)-but-2-enal (3Z)-4-hydroxypent-3-enoic acidarrow_forwardDraw the keto and enol forms of (a) propanal and (b) 3-pentanone.arrow_forwardWrite the reagent or draw structures of the starting material or organic product(s) in the following reactions. If more than one product is formed, identify the major product where possible. (a) (b) HO OH OH H2SO4 ? Cl₂ ? FeCl3arrow_forward
- Part 4: Draw the following molecules. MOST are thiols and phenols. Draw: 3-octanethiol Draw: 2,3-diiodophenol Draw: 1-ethoxy-2-butanethiol Draw: 2-methylbenzenethiol Draw: 1,2-dimethyl 3-chloro-5-propylphenol Draw: 3-cyclobutylphenolarrow_forwardWhat is the major organic product of the following reaction? (a) (b) NaBH4 CH3CH₂OH ? (c) (d) OHarrow_forward1: Give at least five (5) uses of Alcohol and Phenol Name Functional Group R-OH Alcohols LOH Phenols Based on the illustration above. What is the difference between alcohol and phenol? Can Phenol react with alcohol?arrow_forward
- compound H CH, — N — С — СН — СН, || amide - CH, — CH, — с — СH, - - CH3 – CH = CH - CHarrow_forwardDraw the structure of an alkane with molecular formula C7H16 that contains (a) one 4° carbon; (b) only 1° and 2° carbons; (c) 1°, 2°, and 3° hydrogens.arrow_forward3) Beginning from acetylene and any alkyl halide needed, how would you prepare the following compound, there may be more than one step. HC=CH CH;CH,CH,CH,CH,CH,CH;arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License