
(a)
Interpretation:
The stereo isomeric product should be given when the
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2
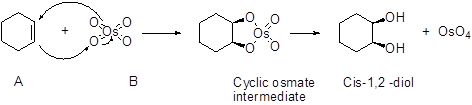
(b)
Interpretation:
The stereo isomeric product should be given when the alkenes reaction with osmium tetroxide followed by hydrogen peroxide hydrolysis.
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2 diol for example,
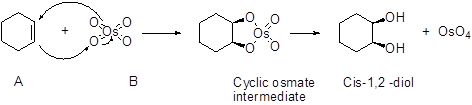
(c)
Interpretation:
The stereo isomeric product should be given when the alkenes reaction with osmium tetroxide followed by hydrogen peroxide hydrolysis.
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2 diol for example,
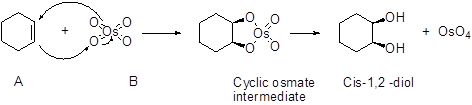
(d)
Interpretation:
The stereo isomeric product should be given when the alkenes reaction with osmium tetroxide followed by hydrogen peroxide hydrolysis.
Concept introduction:
An alkene undergoes an oxidation reaction with Osmium tetra oxide and followed by hydrolysis to give a cis 1, 2 diol for example,
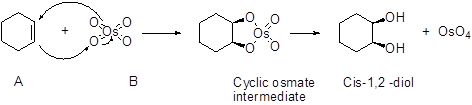
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Organic Chemistry
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

