
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11.7, Problem 25P
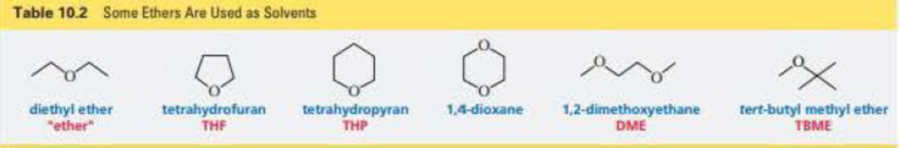
Would you expect the reactivity of a five-membered ring ether such as tetrahydrofuran (Table 10.2) to be more similar to the reactivity of an
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show work with explanation needed. don't give Ai generated solution
Show work. don't give Ai generated solution
None
Chapter 11 Solutions
Organic Chemistry
Ch. 11.1 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 11.1 - Prob. 2PCh. 11.1 - Prob. 5PCh. 11.2 - Prob. 7PCh. 11.3 - Prob. 9PCh. 11.3 - Show how 1-propanol can be converted into the...Ch. 11.4 - Which of the following alcohols dehydrates the...Ch. 11.4 - Prob. 12PCh. 11.4 - Prob. 13PCh. 11.4 - Propose a mechanism for each of the following...
Ch. 11.4 - Draw the product of each of the following...Ch. 11.4 - Explain why the following alcohols, when heated...Ch. 11.4 - What stereoisomers are formed in the following...Ch. 11.4 - Prob. 18PCh. 11.4 - What alcohol would you treat with phosphorus...Ch. 11.5 - Prob. 20PCh. 11.6 - Prob. 22PCh. 11.7 - Prob. 24PCh. 11.7 - Would you expect the reactivity of a five-membered...Ch. 11.7 - Prob. 26PCh. 11.7 - What products are obtained from the reaction of...Ch. 11.7 - Prob. 28PCh. 11.7 - Prob. 29PCh. 11.7 - Prob. 30PCh. 11.8 - Prob. 31PCh. 11.8 - Prob. 32PCh. 11.8 - How do the major products obtained from...Ch. 11.8 - Explain why the two arene oxides in Problem 38...Ch. 11.8 - Three arene oxides can be obtained from...Ch. 11.9 - Explain why the half-life (the time it takes for...Ch. 11.10 - Prob. 38PCh. 11.10 - Prob. 39PCh. 11.10 - Prob. 40PCh. 11.10 - Prob. 41PCh. 11.10 - Prob. 42PCh. 11.11 - Using an alkyl halide and a thiol as starting...Ch. 11.11 - The following three nitrogen mustards were studied...Ch. 11.11 - Why is melphalan a good cancer drug?Ch. 11.11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Which compound is more likely to be carcinogenic?Ch. 11 - Prob. 50PCh. 11 - Prob. 51PCh. 11 - Write the appropriate reagent over each arrow.Ch. 11 - What alkenes would you expect to be obtained from...Ch. 11 - Prob. 54PCh. 11 - When heated with H2SO4, both...Ch. 11 - What is the major product obtained from the...Ch. 11 - When deuterated phenanthrene oxide undergoes a...Ch. 11 - An unknown alcohol with a molecular formula of...Ch. 11 - Prob. 59PCh. 11 - Prob. 60PCh. 11 - Propose a mechanism for the following reaction:Ch. 11 - What product would be formed if the four-membered...Ch. 11 - Which of the following ethers would be obtained in...Ch. 11 - Using the given starting material any necessary...Ch. 11 - Prob. 65PCh. 11 - When 3-methyl-2-butanol is heated with...Ch. 11 - Propose a mechanism for each of the following...Ch. 11 - How could you synthesize isopropyl propyl ether,...Ch. 11 - When the following seven-membered ring alcohol is...Ch. 11 - Ethylene oxide reacts readily with HO because of...Ch. 11 - Prob. 71PCh. 11 - Propose a mechanism for each of the following...Ch. 11 - Explain why the acid-catalyzed dehydration of an...Ch. 11 - Triethylene glycol is one of the products obtained...Ch. 11 - Prob. 75PCh. 11 - Prob. 76PCh. 11 - When ethyl ether is heated with excess HI for...Ch. 11 - Propose a mechanism for the following reaction:Ch. 11 - Prob. 79PCh. 11 - An ion with a positively charged nitrogen atom in...Ch. 11 - Propose a mechanism for each of the following...Ch. 11 - Prob. 82PCh. 11 - The following reaction takes place several times...Ch. 11 - A vicinal diol has OH groups on adjacent carbons....Ch. 11 - Prob. 85PCh. 11 - Prob. 86PCh. 11 - Two stereoisomers are obtained from the reaction...Ch. 11 - Propose a mechanism for each or the following...Ch. 11 - Triethylenemelamine (TEM) is an antitumor agent....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY