
Concept explainers
(a)
Interpretation:
The major products formed under the given reaction conditions should be identified.
Concept introduction:
SN1 reaction:
The alcohols reacts with acids like hydrochloric acid or hydrobromic acid which yield the corresponding carbocation intermediate, this carbocation intermediate undergoes substitution reaction which yields the corresponding substitution product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
Primary alcohol is less stable and won’t undergo
(b)
Interpretation:
The major products formed under the given conditions should be identified.
Concept introduction:
SN2 reaction:
The alcohols reacts with acids like hydrochloric acid or hydrobromic which yield the corresponding substitution product. Primary alcohol undergoes
(c)
Interpretation:
The major products formed under the given conditions should be identified.
Concept introduction:
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid.
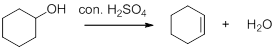
The stability of carbocation is given below,
Tertiary carbocation is more stable than the secondary and primary.
(d)
Interpretation:
The major products formed under the given conditions should be identified.
Concept introduction:
Chromic Acid:
Chromic Acid (
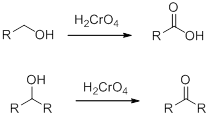
(e)
Interpretation:
The major products formed under the given conditions should be identified.
Concept introduction:
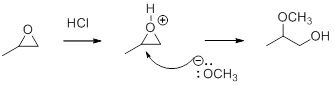
In the presence of acid catalyst, this reaction takes place through partial SN1 and partial SN2 pathway.
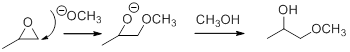
Epoxides are reactive, methoxide ion attacks the Epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product.
(f)
Interpretation:
The major products formed under the given conditions should be identified.
Concept introduction:

In the presence of acid catalyst, this reaction takes place through partial SN1 and partial SN2 pathway. Epoxides are reactive, Epoxides get protonated followed by alcohol attacks to the stable carbocation and form the product.
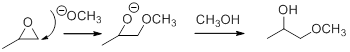
Epoxides are reactive, methoxide ion attacks the Epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product.
(g)
Interpretation:
The major products formed under the given conditions should be identified.
Concept introduction:
Tosylation reaction:
The alcohol is treated with any tosyl chloride (methane sulfonyl chloride) which yields tosylated product this reaction is called as alkyl tosylate and which is shown below,
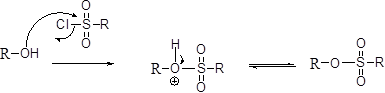
SN2 reaction:
The alcohols is reaction with acids like hydrochloric acid or hydrobromic acid, the bromine atom attacks back side of the carbon atoms which is bearing alcohol group which yield the corresponding inversion product.
(h)
Interpretation:
The major products formed under the given conditions should be identified.
Concept introduction:
Oxidation of alcohol:
Alcohols reaction with hypochlorous (oxidizing agent) in the presence of acetic acid which yields the corresponding
Primary alcohols gives aldehyde, secondary alcohols gives ketone.
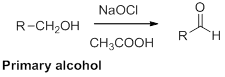
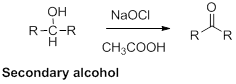
(i)
Interpretation:
The major products formed under the given conditions should be identified.
Concept introduction:
Generally
For elimination reaction quartnary ammonium halide can be converted in to quartnary ammonium hydroxide by using aqueous silver oxide.
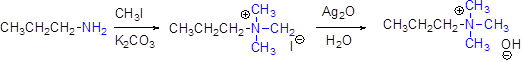
Hofmann elimination:
Quartnary ammonium ion undergoes elimination when using strong base like hydroxide ion this reaction is called as hofmann elimination. Proton abstraction is takes place in β- carbon atom which is having more number of hydrogen.

(j)
Interpretation:
The major products formed under the given conditions should be identified.
Concept introduction:
SN1 reaction: It is a nucleophilic substitution reaction in which the rate determining step depends on one reactant. First step is the formation of more stable carbocation which is followed by the attack of nucleophile. Formation of more stable carbocation and the leaving group present in the substrate plays very important role in the reactivity of
The alcohols is reaction with acids like hydrochloric acid or hydrobromic which yield the corresponding carbocation intermediate, this carbocation intermediate undergoes substitution reaction which yields the corresponding substitution product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
Primary alcohol is less stable therefore it won’t undergoes SN1 substitution reaction
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Organic Chemistry
- The complex anion in Ba₂[Cr(CN)6] is a tetragonally distorted octahedral complex (Dan). Baz[Cr(CN)6] is paramagnetic at room temperature with S = 1. Assume that the complex is a low-spin complex. a) Identify if the [Cr(CN)6] anionic complex has 4 long and 2 short bonds (left side of figure) or if the complex has 4 short and 2 long bonds (right side of figure) with respect to Oh symmetry. Use crystal field theory to answer this question. Explain/rationalize your decision. Can the provided information decide on the order of orbital energies? Dah Tetragonal Distortion ய Dab z-compression z-elongation x and y elongation O symmetry x and y compression E eg d² dx²-y² t2g dxy dxz dyz Question 4 a) continued: Provide your explanations in the space below. b) At low temperatures Ba₂[Cr(CN)6] is ferromagnetically ordered with a phase transition to a paramagnetic phase at Tc = 150K. Sketch the magnetic susceptibility vs. temperature in the diagram below. Indicate Tc as well as the paramagnetic and…arrow_forwarda) Draw the octahedral mer-[FeCl3(CN)3] complex and determine its point group. Use proper wedges and dashes in order to illustrate 3 dimensional details. Use the point group to determine if the complex has a resulting net dipole moment and describe its allowed direction with respect to its symmetry elements (if applicable). ード M 4- b) Substitute one chlorido ligand in mer-[FeCl3(CN)3] 4 with one fluorido ligand. Determine all possible isomers and their corresponding point groups. Use the point groups to determine if the complexes have resulting net dipole moments and describe their allowed direction with respect to its symmetry elements (if applicable). The number of complex sketches below is not necessarily indicative of the number of isomers. 4- 4- ☐☐☐ c) Substitute two chlorido ligands in mer-[FeCl3 (CN)3] 4 with two fluorido ligands. Determine all possible isomers and their corresponding point groups.. Use the point groups to determine if the complexes have resulting net dipole…arrow_forwardShow work. don't give Ai generated solutionarrow_forward
- Differentiate electron spin and electron spin moment.arrow_forwardDifferentiate between nuclear spin and electron spin.arrow_forwardDraw the trigonal prismatic MH6 molecular compound, where M is a 3d transition metal. a) Draw the trigonal prismatic MH6 molecular compound and determine its point group. b) i. What is the symmetry species for the 4s orbital on the central metal? ii. What is the symmetry species for the 3dx²-y² orbital on the central metal? Note: The z-axis is the principal axis. iii. Suggest a crystal field energy diagram for a d² electron configuration in a trigonal prismatic coordination environment. Label the metal d-orbital with their corresponding symmetry species label. Use the appropriate character table in the resource section.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





