
Without referring to your textbook or a periodic table, write the full electron configuration, the orbital box diagram, and the noble gas shorthand configuration for the elements with the following
msp;
(a)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 1.
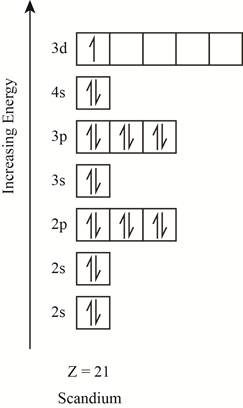
Figure 1
The electronic configuration of the given element can also be written as
(b)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 2.
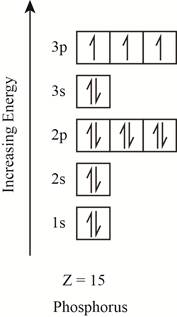
Figure 2
The electronic configuration of the given element can also be written as
(c)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 3.
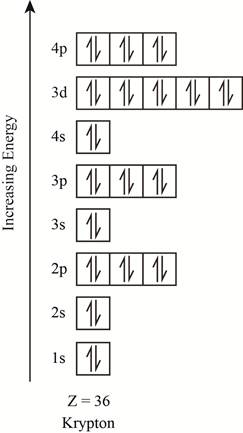
Figure 3
The electronic configuration of the given element can also be written as
(d)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 4.
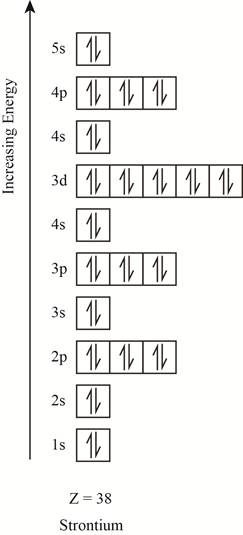
Figure 4
The electronic configuration of the given element can also be written as
(e)
Interpretation:
The electronic configuration of the given element, the orbital box diagram and the noble gas shorthand configuration for the elements are to be stated.
Concept Introduction:
The distribution of the electrons that exists in the atomic orbital of an atom is collectively known as electronic configuration. The description of every electron in an orbital is given by the electronic configuration of that atom.
Answer to Problem 97AP
The electronic configuration of the given element with
Explanation of Solution
The electronic configuration of the given element with
The orbitals in the orbital box diagram are arranged in increasing order of energy shells. The orbital box diagram is shown in figure 5.
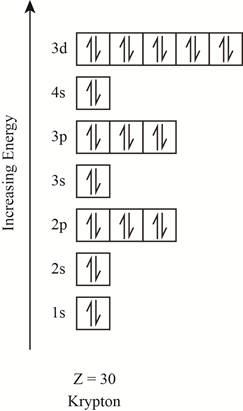
Figure 5
The electronic configuration of the given element can also be written as
Want to see more full solutions like this?
Chapter 11 Solutions
Introductory Chemistry: A Foundation
- K Problem 16 of 24 Submit Draw the starting structure that would yield this product under these conditions. Select to Draw 1. NH4Cl, NaCN 2. HCI, H2O, A NH3 + 0arrow_forwardGive detailed me detailed mechanism Solution with explanation needed. Don't give Ai generated solution. avoid handwritten Solutionarrow_forwardShow work with explanation needed. don't give Ai generated solutionarrow_forward
- K Problem 21 of 24 Submit Draw the missing organic structures in the following multistep synthesis. Show the final product at physiological pH (pH = 7.4). Ignore any inorganic byproducts formed. H 0 NH3 Select to Draw HCN H+, H2O Select to Draw Select to Draw Δarrow_forwardShow work with explanation needed. Don't give Ai generated solution. Give correct solutionarrow_forwardK Problem 23 of 24 Submit Draw the product of the reaction shown below at physiological pH (pH = 7.4). Ignore inorganic byproducts. S O 1. NH3, 2. HCN 3. H+, H₂O, A Select to Drawarrow_forward
- Please correct answer and don't use hand rating and don't use Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward14. Draw all of the products expected for the following reaction. Circle the products expected to predominate when the reaction is heated to 40 °C. EXPLAIN your choice. (12 points) HBr ? Br -11arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





