
Concept explainers
Interpretation: To propose a plausible synthesis for
Concept introduction: The retrosynthesis begins with disconnection at the ether groups, at two of the
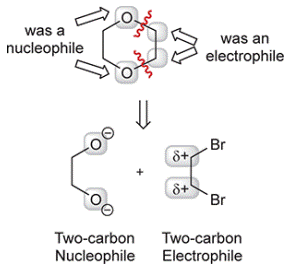
Each of these starting materials can be made from acetylene.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Alkenes can be converted into alcohols by acid-catalyze addition of water. Predict the major alcohol product from this alkene below.arrow_forward1. Predict the product, and propose a mechanism for the following nucleophilic addition to aldehyde or ketone. Are the products different from one another? Why do the reactions proceed via different mechanisms? Note the charges on the intermediates of acid and base catalyzed reactions. Oxygen nucleophile in acidic condition H2O, [H+] Oxygen nucleophile in basic condition NaOHarrow_forwardWrite an equation for the acid-base reaction between 2,4-pentanedione and sodium eth- oxide and calculate its equilibrium constant, K. The pK, of 2,4-pentanedione is 9; that of ethanol is 15.9. CH,CCHÖCH, + CH,CH,O Na* H 2,4-Pentanedione Sodium ethoxidearrow_forward
- O NaBH4 Synthesis #2: Select a different set of two substrates and one reagent that could be combined to prepare benzphetamine. tymen Ph Ph Ph Ph NH Ph H O NaBH3CN, H+ 20= ano [H*], NaBH-CN Nucleophilearrow_forwardOOn a reaction with acid, 4-pyrone is protonated on the carbonyl-group oxygen to give a stable cationic product. Using resonance structures and the Huckel 4n+2 rule, explain why the protonated product is so stable. 4-Pyronearrow_forward2 By using the same alkene, how do you synthesize the following alcohols? Give the alkene and show the mechanisms for each product formation. OH OH НО.arrow_forward
- Propose a structure for an aromatic hydrocarbon, C10H14, that can form only one C10H13Cl product on substitution of a hydrogen on the aromatic ring with chlorine.arrow_forwardCH3 གོན་ནི། ཡོན་ Br₂, H₂C CH₂Br H3C CH3 H₂C CH3 Aldehydes and ketones can be halogenated at their a-position by reaction with Cl₂, Br2, or 12, under acidic conditions. Using Br₂ under acidic conditions, an intermediate enol is formed which adds bromine at the a-position. The reaction stops after the addition of one bromine because the electron-withdrawing halogen decreases the basicity of the carbonyl oxygen, making the protonation less favorable. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions :0: :OH н-он H-OH₂ H₂O H₂C. H3C. CH3 CH3 H3C CH3 H3C CH3 Σαarrow_forwardStarting with benzene and using any other reagents of your choice, design a synthesis for the following compound: Br XX O₂N The target molecule above can be prepared by treating benzene with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A HNO3, H₂SO4 D Fuming H₂SO4 B E Br2, AlBr3 Dilute H₂SO4 C BrMg F MgBr axxa. AICI 3arrow_forward
- Write the Ky reactions for pyridine, for sodium 2-mercaptoethanol, and for cyanide. H+ binds to S in sodium 2-mercaptoethanol and to C in cyanide. (Hint: Spectator ions, which do not react in solution, appear on both sides of the reaction and can be removed.) N: HOCH.CHS: Na+ CN Pyridine Sodium 2-mercaptoethanol Cyanide Refer to the Ka values below for their conjugate acids. Which of the three bases shown above is the strongest? NH HOCH.CH.SH HCN Pyridinium ion K. = 6.3 x 10-6 2-Mercaptoethanol K. = 1.9 X 10-10 Hydrogen cyanide K = 6.2 x 10-10arrow_forwardi) ii) 1. Propose a mechanism for the following reactions. H (CH3)2NH H* NaOH EtOHarrow_forwardStarting from acetylene and alkyl halides not longer than 4 carbon atoms, show the synthesis of 3-octanol and a ketone. Indicate the reagents in each step.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

