
(a)
Interpretation:
Line angle formula to be identified for 2,2,4-trimethylhexane.
Concept Introduction:
Example of
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 19P
The line angle formula for 2,2,4-trimethylhexane.
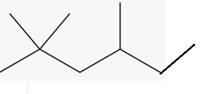
Explanation of Solution
The line angle formula for 2,2,4-trimethylhexane.
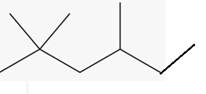
(b)
Interpretation:
Line angle formula to be identified for 2,2-dimethylpropane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 19P
The line angle formula for 2,2-dimethylpropane.

Explanation of Solution
The line angle formula for 2,2-dimethylpropane.

(c)
Interpretation:
Line angle formula to be identified for 3-ethyl-2,4,5-trimethyloctane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 19P
The line angle formula for 3-ethyl-2,4,5-trimethyloctane.
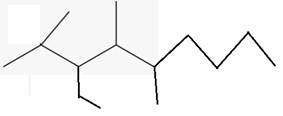
Explanation of Solution
The line angle formula for 3-ethyl-2,4,5-trimethyloctane.
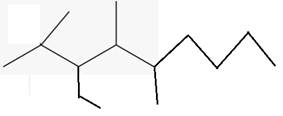
(d)
Interpretation:
Line angle formula to be identified for 5-butyl-2,2-dimethylnonane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 19P
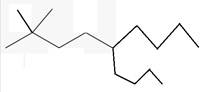
The line angle formula for 5-butyl-2,2-dimethylnonane.
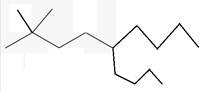
Explanation of Solution
The line angle formula for 5-butyl-2,2-dimethylnonane.
(e)
Interpretation:
Line angle formula to be identified for 4-isopropyloctane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 19P
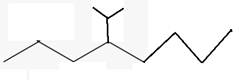
The line angle formula for 4-isopropyloctane.
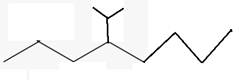
Explanation of Solution
The line angle formula for 4-isopropyloctane.
(f)
Interpretation:
Line angle formula to be identified for 3,3-dimethylpentane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 19P
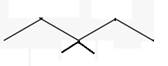
The line angle formula for 3,3-dimethylpentane.
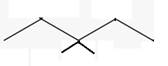
Explanation of Solution
The line angle formula for 3,3-dimethylpentane.
(g)
Interpretation:
Line angle formula to be identified for trans-1,3-dimethylcyclopentane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 19P
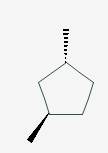
The line angle formula for trans-1,3-dimethylcyclopentane.
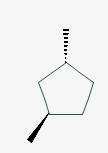
Explanation of Solution
The line angle formula for trans-1,3-dimethylcyclopentane.
(h)
Interpretation:
Line angle formula to be identified for Cis-1,2-diethylcyclobutane.
Concept Introduction:
Example of alkane are propane having formula C3 H8, butane having formula C4 H10 etc. Their molecular formula indicate the number of hydrogen is 2 more than twice the number of carbon in the structure and thus the derived formula for alkane is Cn H2n +2.
In line angle formula each line indicates 2 carbon are linked to each other through a single bond.
Answer to Problem 19P
The line angle formula for Cis-1,2-diethylcyclobutane.
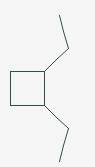
Explanation of Solution
The line angle formula for Cis-1,2-diethylcyclobutane.
Want to see more full solutions like this?
Chapter 11 Solutions
Introduction to General, Organic and Biochemistry
- Indicate the correct answer.a) In boranes, the B-B bonds are the most reactive.b) The B-H-B bonds are the reactive centers in the B2H6 molecule.arrow_forwardIn boranes, the B-B bonds are the most reactive.arrow_forwardThe B-H-B bonds are the reactive centers in the B2H6 molecule. Correct?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





