
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 52P
For each of the following pairs of compounds, identify one IR absorption band that could be used to distinguish between them:
- a. CH3CH2CH2OH and CH3CH2OCH3
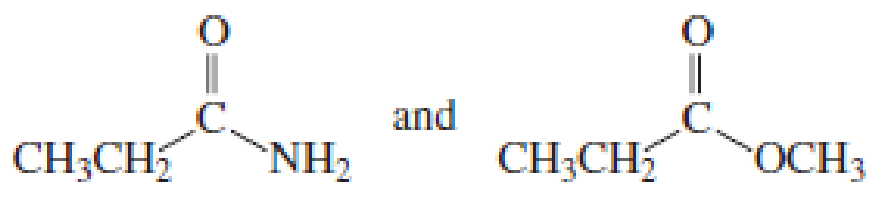
- b. CH3CH2CH = CHCH3 and CH3CH2C − CCH3
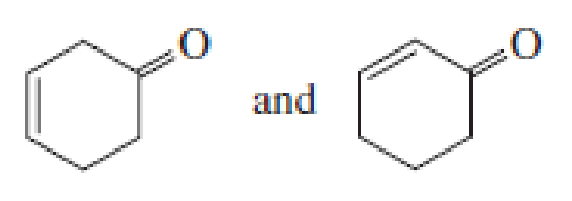
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2. Compound B has a molecular formula of C₁H₁2O₂. It has a characteristic IR signal at
around 1700 cm³¹. Draw the molecular structure of B.
3H
1H
1H 1H
L
200
180
160 140
PPM
120
PPM
100
2H 2H
80
60
2H
40
20
How many different 13C-absorption lines and how many 'H-
resonances (disregard splitting and assume that solvent exchange of acidic
hydrogens does NOT take place) are observed in the spectrum of each of
the following compounds?
(H3C)3C-C(CH3)2
CI
A
H3C
(H3C)3C
B
CH3
CH3
H₂C=
-COOH
C
CH-CI
H3C-C-CH
Cl Br
D
CH3
c=C
H3C
H.
Chapter 10 Solutions
Essential Organic Chemistry, Global Edition
Ch. 10.1 - Prob. 1PCh. 10.2 - What would distinguish the mass spectrum of...Ch. 10.2 - Prob. 3PCh. 10.3 - Prob. 5PCh. 10.3 - Suggest possible molecular formulas for a compound...Ch. 10.3 - Prob. 7PCh. 10.4 - Prob. 8PCh. 10.4 - Prob. 9PCh. 10.5 - Prob. 10PCh. 10.5 - Prob. 11P
Ch. 10.6 - Identify the ketone responsible for the mass...Ch. 10.6 - Prob. 13PCh. 10.8 - Prob. 14PCh. 10.8 - Prob. 15PCh. 10.12 - Which will occur at a larger wavenumber: a. a C :...Ch. 10.13 - Which will occur at a larger wavenumber: a. the C...Ch. 10.13 - Prob. 18PCh. 10.13 - Prob. 19PCh. 10.13 - Which will show an O 8 H stretch at a larger...Ch. 10.14 - Prob. 21PCh. 10.14 - Prob. 22PCh. 10.15 - Prob. 23PCh. 10.15 - Prob. 24PCh. 10.17 - Prob. 25PCh. 10.18 - Prob. 26PCh. 10.18 - Prob. 27PCh. 10.19 - Prob. 28PCh. 10.19 - Prob. 29PCh. 10.22 - How many signals would you expect to see in the 1H...Ch. 10.22 - Prob. 31PCh. 10.22 - Prob. 32PCh. 10.23 - Where would you expect to find the 1H NMR signal...Ch. 10.24 - Prob. 34PCh. 10.25 - Prob. 35PCh. 10.25 - Prob. 36PCh. 10.25 - Prob. 37PCh. 10.26 - Prob. 38PCh. 10.26 - Which of the following compounds is responsible...Ch. 10.27 - Prob. 40PCh. 10.27 - Prob. 41PCh. 10.27 - The 1H NMR spectra of two carboxylic acids with...Ch. 10.28 - Prob. 43PCh. 10.28 - Prob. 44PCh. 10.28 - Prob. 45PCh. 10.28 - Describe the 1H NMR spectrum you would expect for...Ch. 10.28 - Identify the compound with molecular formula...Ch. 10.29 - Prob. 48PCh. 10.29 - Prob. 49PCh. 10.29 - Identify the compound with a molecular formula of...Ch. 10 - In the mass spectrum of the following compounds,...Ch. 10 - For each of the following pairs of compounds,...Ch. 10 - Draw the structure of a saturated hydrocarbon that...Ch. 10 - Prob. 54PCh. 10 - Prob. 55PCh. 10 - How could you use UV spectroscopy to distinguish...Ch. 10 - Prob. 57PCh. 10 - Predict the relative intensities of the molecular...Ch. 10 - Prob. 59PCh. 10 - List the following compounds in order from highest...Ch. 10 - How can 1H NMR be used to prove that the addition...Ch. 10 - There are four esters with molecular formula...Ch. 10 - Prob. 63PCh. 10 - Prob. 64PCh. 10 - Each of the IR spectra presented here is...Ch. 10 - Prob. 66PCh. 10 - Five compounds are shown for each of the following...Ch. 10 - Prob. 68PCh. 10 - Prob. 69PCh. 10 - Phenolphthalein is an acidbase indicator. In...Ch. 10 - Which one of the following five compounds produced...Ch. 10 - Prob. 72PCh. 10 - Prob. 73PCh. 10 - Prob. 74PCh. 10 - How could 1H NMR distinguish between the compounds...Ch. 10 - Prob. 76PCh. 10 - Prob. 77PCh. 10 - The 1H NMR spectra of three isomers with molecular...Ch. 10 - The 1H NMR spectra of three isomers with molecular...Ch. 10 - Identify the following compounds. (Relative...Ch. 10 - An alkyl halide reacts with an alkoxide ion to...Ch. 10 - Determine the structure of a compound with...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 14. Compound B has molecular formula C9H12. It shows five signals in the 1H-NMR spectrum - a doublet of integral 6 at 1.22 ppm, a septet of integral 1 at 2.86 ppm, a singlet of integral 1 at 5.34 ppm, a doublet of integral 2 at 6.70 ppm, and a doublet of integral 2 at 7.03 ppm. The 13C-NMR spectrum of B shows six unique signals (23.9, 34.0, 115.7, 128.7, 148.9, and 157.4). Identify B and explain your reasoning.arrow_forward15) Which compound does not have a strong, characteristic IR absorption near 1700 cm-1? B) (CH3CH2)20 C) CH3CH2CHO D) (CH3CH2)2C=Oarrow_forwardCH3CH2 -OH CH3- -OCH3 B -OCH2CH2CH3 C Which compound, if any, can be distinguished from the others by the molecular ions in their mass spectra? B A ◇ None, they all have the same molar mass. осarrow_forward
- 1. Factors determining intensity and energy level of absorption in IR spectra. What features will be the similar in the IR spectra of the following compounds, and how will their IR spectra differ? CH3C(O)(CH₂)3OH and CH3CH₂C(O)(CH₂)2OHarrow_forward1. Determine the stereochemical relationship (homotopic, enantiotopic, diastereotopic) between each pair of hydrogens (a and b) in the molecules represented below. Also, determine the number of signals that would be present in the ¹H NMR spectrum of each molecule. Stereochemical Relationship Structure Ha "H₂ Ha 3 Spectroscopy Hb Ha # of Signalsarrow_forwardChemistry Amide groups have a uniquely low-energy C=O stretch; it can be easily observed by IR spectroscopy. Which of the following amide C=O stretches has the higher absorption value in wavenumbers (cm ¹) and why? NO₂ ly so 'N 1 2 2 because the CO double bond is weaker 2 because the CO double bond is stronger 1 because the CO double bond is weaker 1 because the CO double bond is strongerarrow_forward
- Which of the following compounds has a vibration that is infrared inactive?1-butyne, 2-butyne, H2, H2O, Cl2, and ethenearrow_forwardIdentify the structures of isomers H and I (molecular formula C&HuN). a. Compound H: IR absorptions at 3365, 3284, 3026, 2932, 1603, and 1497 cm 2H 5 H 2H 2H 0 2 3 4 5 6 7 ppm b. Compound I: IR absorptions at 3367, 3286, 3027, 2962, 1604, and 1492 cm1 3 H 5 H 1 H 2 H 3 2 8 5 4 7arrow_forwardWhat IR absorption would you use to distinguish between the two compounds below? a CH3CCH₂CH3 O 2850-2960 1715 3400-3650 O2210-2260 A and CH3 b CH3CHCH₂CH3arrow_forward
- How do the three isomers of molecular formula C3H6O (A, B, and C) differ in their IR spectra?arrow_forward3. Consider the isomers A, B and C given below: CH3 CH3 CH3 Br в A Br Br 3-Bromomethylbenzene 4-Bromomethylbenzene 2-Bromomethylbenzene (c) For each compound how many chemically different carbon signals would you expect to see in their 1°C NMR spectra? B = C = A =arrow_forwardConsider a molecule with the molecular formula of C3H7NO2. The IR and ¹H NMR spectra are shown below. Draw the structure that best fits this data. 4000 3000 2H 2000 1500 Wavenumbers (cm-¹) + 2H 1000 3H 500 ppmarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY