
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 8E
a model of each of the following molecules: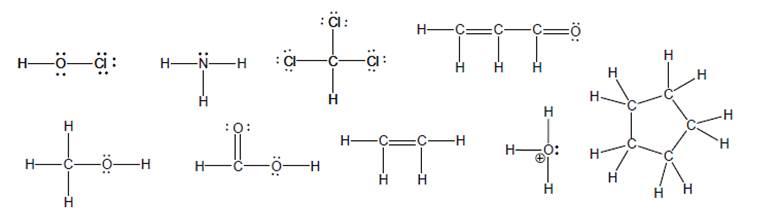
a. Based on your model, draw a bond-line representation with as many atoms as possible in the plane of the paper. Use wedge and dash bonds to represent any atoms that do not lie in the plane of the paper.
b. Indicate each unique bond angle and the shape of each unique central atom.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which statement A-D about VSEPR theory is not correct?
Select one:
a. The molecular shape or geometry can differ from the electron-pair geometry.
b. In VSEPR theory, the shape or geometry of a molecule is determined by electron-electron repulsion.
c. The steric number of a central atom is the sum of the number of bonded atoms and lone pairs around the central atom.
d. The steric number has five values from 2 to 6.
e. Statements A-D are all correct.
Clear my choice
Which statement about VSEPR theory is not correct?
Select one:
a. The molecular shape or geometry is determined by the positions of the lone pairs in the molecule.
b. The steric number has five values from 2 to 6.
c. The steric number of a central atom is the sum of the number of bonds around the atom plus the number of electrons in lone pairs pairs
d. The electron-pair geometry is determined by the positions of the bonds and lone pairs in a molecule.
e. In VSEPR theory, the shape or geometry of a molecule is determined by electron-electron repulsion.
Answer each of the following questions correctly. Choose the correct answer.
1. It is a measure of how equally the electrons in a bond are distributed between the two atoms involved in a covalent bond.
a. Polarity b. Octet rule
c. Ionization energy d. Electron affinity
2. The shape of bonding molecular orbital shows that the greatest electron density is in the region.
a. Between the two nuclei
b. Close to the more atom electronegative
c. Close to the bigger atom
d. Uniformly around the two nuclei
3. In which compound is the bond that has the most ionic character found?
a. HCl b. Kl c. MgS d. NO
Chapter 1 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 1 - (E) What does the number (+Z) at the center of...Ch. 1 - Prob. 2CTQCh. 1 - Prob. 3CTQCh. 1 - Prob. 4CTQCh. 1 - Prob. 5CTQCh. 1 - Prob. 6CTQCh. 1 - Prob. 7CTQCh. 1 - You hear a student from a nearby group say that...Ch. 1 - Use VSEPR to explain why the HBH bond angle of BH3...Ch. 1 - Both the HCH and HCO bond angles of H2CO...
Ch. 1 - Prob. 11CTQCh. 1 - Consider the following flat drawing of methane...Ch. 1 - Use VSEPR to assign a value of (close to) 109.5,...Ch. 1 - A student draws the picture of ammonia (NH3) in...Ch. 1 - Prob. 15CTQCh. 1 - How many central atoms does the molecule H2NCH3...Ch. 1 - Indicate the bond angle and shape about each...Ch. 1 - Explain how there can be two kinds of bent:...Ch. 1 - A student makes the following statement: “The...Ch. 1 - A student who missed this class needs to know how...Ch. 1 - Prob. 1ECh. 1 - Prob. 2ECh. 1 - Consider the incomplete valence shell...Ch. 1 - How many valence electrons does a neutral a. K...Ch. 1 - Consider the molecules AlCl3 (aluminum chloride)...Ch. 1 - Draw an example of a bent molecule with a bond...Ch. 1 - Label each atom marked with an arrow with the...Ch. 1 - a model of each of the following molecules: a....
Additional Science Textbook Solutions
Find more solutions based on key concepts
Which of the following solutions has the higher molarity? 10 ppm KI in water or 10,000 ppb KBr in water 0.25 ma...
CHEMISTRY-TEXT
Real walls are never totally adiabatic. Use your experience to order the following walls in increasing order wi...
Thermodynamics, Statistical Thermodynamics, & Kinetics
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (14th Edition)
Draw a Lewis structure for each of the following species: a. H2CO3 b. CO32 c. CH2O d. CO2
Essential Organic Chemistry (3rd Edition)
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Considering the position of the elements in the periodic table and their relative electronegativities and bond polarities, which bond is longest? a. carbon - Oxygen triple bond b. carbon - Oxygen single bond c. carbon - Carbon single bond d. carbon - Carbon double bond e. carbon - Nitrogen triple bond Which bond is the strongest? a. carbon - Nitrogen triple bond b. carbon - Nitrogen double bond c. carbon - Hydrogen bond d. carbon - Carbon triple bond e. carbon - Carbon single bondarrow_forwardSHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 1. Is it possible for a molecule to be nonpolar even though it contains polar bonds? Explain your answer and give an example.arrow_forward2. This question uses sulfur trioxide and the sulfite ion and sulfur trioxide a. Draw both Lewis structures. b. Give the shape and geometry of each structure.arrow_forward
- Which statement A-D about VSEPR theory is not correct? Select one: a. The steric number has five values from 2 to 6. b. Statements A-D are all correct. c. In VSEPR theory, the shape or geometry of a molecule is determined by electron-electron repulsion. d. The steric number of a central atom is the sum of the number of bonds and lone pairs around the atom. e. The molecular shape or geometry can differ from the electron-pair geometry.arrow_forward1. Neatly draw the Lewis dot structure. (Must be hand-drawn and very neat) 2. Count and report the number of electron regions around the center atom. 3. Neatly draw the molecule. Include lone pair electrons and use wedges and dotted lines as needed. 4. Report the "electronic geometry" and the "molecular geometry" names. Molecule #1: 13 (note that this -1 anion has one more additional electron) Molecule #2: NH3arrow_forwardA. CHF i. Best Lewis Structure B. HNO (H is connected to one of the O's) i. Best Lewis Structure ii. Electron geometry on the C atom ii. Electron geometry on the N atom iii. Approximate bond angles about the C atom iii. Approximate bond angles around the N atom v. Draw the shape with in and out wedges (as necessary) and dipole arrows around the C atom. v. Draw the shape with in and out wedges (as necessary) and dipole arrows around the N atom. vi. Is the molecule polar or nonpolar? vi. Is the molecule polar or nonpolar?arrow_forward
- 1. Determine whether the following hypothetical compounds are polar or nonpolar. Briefly explain how you can tell. a. A compound with a tetrahedral molecular geometry in which all four bonds are to four atoms of the same element. b. A compound with a tetrahedral molecular geometry in which two of the bonds are polar bonds to two fluorine atoms and two of the bonds are nonpolar bonds to two hydrogen atoms. c. A trigonal pyramidal compound that contains one polar bond. d. A trigonal pyramidal compound that contains only nonpolar bonds. e. A compound in which the central atom has two lone pairs and forms polar bonds to three fluorine atoms. f. A compound in which the central atom has three lone pairs and forms polar bonds to two fluorine atoms.arrow_forwardClassify each molecule as polar or nonpolar. Electrons on the outer atoms are omitted for clarity. Polar Nonpolar Answer Bank F F. F F. F F-Xe-F F Farrow_forward1. Draw the best Lewis dot structure for the anion CCl, in the correct molecular geometry (Include formal charges and lone pair electrons, and use dashed and solid wedge bonds if necessary) 2. How many electron groups are present around the central atom and what is the electron group geometry? 3. What is the molecular geometry and ideal bond angles? 4. Is the molecule polar or honpolar? If it is polar, draw a dipole moment arrow next to your structure to indicate the directionality of the dipole moment. Answers: Edit View Insert Formuat Tools Table 12pt Paragraph BIUA 2 of Bons O words IMG4Sg IMG 3449 og IMG 344 jpg IMG 3447 jp Dearrow_forward
- Chemistry Which of the following bond would (theoretically) take the most energy to disrupt? Select one: O a. the bond between a sodium atom and a chlorine atom O b. the bond between the oxygen and the hydrogen on a fatty acid O c. the bond between on oxygen atom and a carbon atom on a fatty acid O d. the bond between a carbon and carbon on a fatty acid e. the bond between a carbon and an oxygen on a carbohydrate a is not a correct answer, so it's either b,c,d, or e.arrow_forwardDraw a Lewis structure for NH3 and answer the following questions based on your drawing. 1. For the central nitrogen atom: The number of lone pairs The number of single bonds = The number of double bonds= 2. The central nitrogen atom A. Obeys the octet rule B. Has an incomplete octet. C. Has an expanded octet. =arrow_forwardQuestion 48 48. Which of the following statements are true? 1. 11. III. IV. The electrons in each molecule tend to orient themselves around the most electronegative element. Each molecular drawing follows the localized electron model. Both HF and CO₂ are linear molecules and therefore polar. The bond angles of NH3 are slightly less than 109.5° because the lone pair compresses the angles between the bonding pairs. a. I, III, IV b. I, II, IV c. I, II, III d. II, IV e. All of the above statements are correct. C B esc E Q @ 2 W # 3 E $ 4 * R % 5 T < 6 Y & 7 8 Uarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY