
Concept explainers
Consider the following flat drawing of methane
a. What is
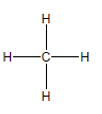
b. Are the electron domains of this flat
c. Use model materials to make a model of
d. In which representation, the drawing above or the model in your hand (circle one) are theH’s of
e. Confirm that your model looks like the following drawing. The wedgebond represents a bond coming out of the page, and the dash bondrepresents a bond going into the page
f. You will often see methane drawn as if it were flat (like on the previous page). Why is thismisleading, and what is left to the viewer’s imagination when looking at such a drawing?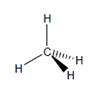
Trending nowThis is a popular solution!

Chapter 1 Solutions
Organic Chemistry: A Guided Inquiry
Additional Science Textbook Solutions
General, Organic, & Biological Chemistry
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
Chemistry
Chemistry: The Central Science (13th Edition)
Fundamentals of Heat and Mass Transfer
- Best Lewis Formula and Molecular Geometry A student writes the Lewis electron-dot formula for the carbonate anion, CO32, as a Does this Lewis formula obey the octet rule? Explain. What are the formal charges on the atoms? Try describing the bonding for this formula in valence bond terms. Do you have any difficulty doing this? b Does this Lewis formula give a reasonable description of the electron structure, or is there a better one? If there is a better Lewis formula, write it down and explain why it is better. c The same student writes the following resonance description for CO2: Is there something wrong with this description? (What would you predict as the geometries of these formulas?) d Is one or the other formula a better description? Could a value for the dipole moment help you decide? e Can you write a Lewis formula that gives an even better description of CO2? Explain your answer.arrow_forwardFormamide, HC(O)NH2, is prepared at high pressures from carbon monoxide and ammonia, and serves as an industrial solvent (the parentheses around the O indicate that it is bonded only to the carbon atom and that the carbon atom is also bonded to the H and the N atoms). Two resonance forms (one with formal charges) can be written for formamide. Write both resonance structures, and predict the bond angles about the carbon and nitrogen atoms for each resonance form. Are they the same? Describe how the experimental determination of the HNH bond angle could be used to indicate which resonance form is more important.arrow_forwardFor each of the following molecules, state the bond angle (or bond angles, as appropriate) that you would expect to see on the central atom based on the simple VSEPR model. Would you expect the actual bond angles to be greater or less than this? a CCl4 b SCl2 c COCl2 d AsH3arrow_forward
- The hyponitrite ion, ONNO, exists in solid compounds as the trans isomer. Using valence bond theory, explain why cistrans isomers might be expected for this ion. Draw structural formulas of the cistrans isomers.arrow_forwardIt is possible to write a simple Lewis structure for the SO42- ion, involving only single bonds, which follows the octet rule. However, Linus Pauling and others have suggested an alternative structure, involving double bonds, in which the sulfur atom is surrounded by six electron pairs. (a) Draw the two Lewis structures. (b) What geometries are predicted for the two structures? (c) What is the hybridization of sulfur in each case? (d) What are the formal charges of the atoms in the two structures?arrow_forwardAmong the following, which has the shortest bond and which has the longest: Li2, B2, C2, N2, O2?arrow_forward
- a Carbonyl fluoride, COF2, is an extremely poisonous gas used in organofluorine synthesis. Give the valence bond description of the carbonyl fluoride molecule. (Both fluorine atoms are attached to the carbon atom.) b Nitrogen, N2, makes up about 80% of the earths atmosphere. Give the valence bond description of this molecule.arrow_forwardAcrylamide, H2C=CHCONH2, is a known neurotoxin and possible carcinogen. It was a shock to all consumers of potato chips and french fries a few years ago when it was found to occur in those products. (a) Sketch the molecular structure of acrylamide and identify all bond angles. (b) Indicate which carbon-carbon bond is the stronger of the two. (c) Is the molecule polar or nonpolar? (d) The amount of acrylamide found in potato chips is 1.7 mg/kg. If a serving of potato chips is 28 g, how many moles of acrylamide are you consuming?arrow_forward32W.) A pi bond is formed by the parallel overlap of s orbitalsX.) Since bromine is from Group VIIA, it needs 7 electrons to complete its octetY.) The bond angle between methane's bond pairs is 109.5⁰Z.) The orbital of f with a shape of y(3x²-y²) contains 14 electronsA.) If all 4 statements are trueB.) If 3 of the 4 statements are trueC.) If 2 of the 4 statements are trueD.) If only 1 of the 4 statements is trueE.) If none of the 4 statements is truearrow_forward
- Consider the following molecules (the number of lone or unshared pairs on the central atom is provided). How many have an electron domain geometry that is tetrahedral? H2O (two lone pairs on O) CCl4 (zero lone pairs on C) SO2 (one lone pair on S) PCl3 (one lone pair on P)arrow_forwardWhat are the angles a and b in the actual molecule of which this is a Lewis structure? H H C H a H C ^ b C a = 11° b=0° H -H Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes. X 3arrow_forwardPredict the relative lengths of the carbon-halogen bonds in CH3F, CH3 Cl, and CH3 Br. Rank from longest to shortest. To rank items as equivalent, overlap them.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





