
Pushing Electrons
4th Edition
ISBN: 9781133951889
Author: Weeks, Daniel P.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 65EQ
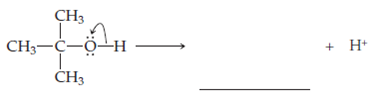
The loss of a proton attached to the oxygen atom of
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Indicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are false
(f) SO:
Best Lewis Structure
3
e group geometry:_
shape/molecular geometry:,
(g) CF2CF2
Best Lewis Structure
polarity:
e group arrangement:_
shape/molecular geometry:
(h) (NH4)2SO4
Best Lewis Structure
polarity:
e group arrangement:
shape/molecular geometry:
polarity:
Sketch (with angles):
Sketch (with angles):
Sketch (with angles):
1.
Problem Set 3b
Chem 141
For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing
bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the
molecule is polar or non-polar (iv)
(a) SeF4
Best Lewis Structure
e group arrangement:_
shape/molecular geometry:
polarity:
(b) AsOBr3
Best Lewis Structure
e group arrangement:_
shape/molecular geometry:
polarity:
Sketch (with angles):
Sketch (with angles):
Chapter 1 Solutions
Pushing Electrons
Ch. 1 - 1. Hydrogen is a Group I element and each...Ch. 1 - Methanol has the molecular formula CH4O. Its...Ch. 1 - 3. The skeleton of chloromethane is...Ch. 1 - 4. Methanol’s skeleton is
Connecting all bonded...Ch. 1 - 5. The structure for chloromethane is
It...Ch. 1 - Prob. 6EQCh. 1 - 7. Dimethyl ether
No. of electrons in...Ch. 1 - Methylamine (CH5N) No. of electrons in structure...Ch. 1 - Methanethiol (CH4S) No. of electrons in structure...Ch. 1 - Methylal (C3H8O2) No. of electrons in structure...
Ch. 1 - Prob. 11EQCh. 1 - Adding electrons to the skeleton by making single...Ch. 1 - This is done by removing an unshared pair from...Ch. 1 - Prob. 14EQCh. 1 - Prob. 15EQCh. 1 - Prob. 16EQCh. 1 - The skeleton of acetyl chloride is . Write the...Ch. 1 - Three constitutional isomers exist for the formula...Ch. 1 - A number of constitutional isomers exist for the...Ch. 1 - Using the method outlined above, derive the...Ch. 1 - Prob. 21EQCh. 1 - Prob. 22EQCh. 1 - Prob. 23EQCh. 1 - Prob. 24EQCh. 1 - The skeleton of benzyldimethylamine is
The...Ch. 1 - The skeleton is benzaldoxime is The number of...Ch. 1 - Prob. 27EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - Prob. 29EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - Prob. 31EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - The Lewis structure of acetone is Circling the...Ch. 1 - Chloromethane has the Lewis...Ch. 1 - In the Lewis structure for chloromethane, the...Ch. 1 - Prob. 36EQCh. 1 - The oxygen atom in acetone possesses ____ unshared...Ch. 1 - Nitrobenzene has the skeleton
The number of...Ch. 1 - Prob. 39EQCh. 1 - Compute and add on the formal charges I these...Ch. 1 - Prob. 41EQCh. 1 - Prob. 42EQCh. 1 - Prob. 43EQCh. 1 - Prob. 44EQCh. 1 - Prob. 45EQCh. 1 - Prob. 46EQCh. 1 - Prob. 47EQCh. 1 - Compute and add on the formal charges in these...Ch. 1 - Prob. 49EQCh. 1 - Prob. 50EQCh. 1 - The n-propyl cation can be formed from a molecule...Ch. 1 - Prob. 52EQCh. 1 - Prob. 53EQCh. 1 - Methanol, CH3OH, is a compound in which the formal...Ch. 1 - When a proton becomes bonded to diethyl ether, by...Ch. 1 - Tetrahydrofuran has the structure
When a proton...Ch. 1 - Prob. 57EQCh. 1 - Prob. 58EQCh. 1 - The structure of pyridine is
When a proton...Ch. 1 - The carbon atom owns one electron from each of ...Ch. 1 - The n-butyl anion can be formed from When the CLi...Ch. 1 - The isobutyl anion can be formed from When the CNa...Ch. 1 - Prob. 63EQCh. 1 - Ethanol, , is a compound in which the formal...Ch. 1 - The loss of a proton attached to the oxygen atom...Ch. 1 - A very strong base can remove a proton from...Ch. 1 - Prob. 67EQCh. 1 - Prob. 68EQCh. 1 - Prob. 69EQCh. 1 - The homolysis of the OO bond in diacetyl peroxide...Ch. 1 - Prob. 71EQCh. 1 - Prob. 72EQCh. 1 - Prob. 73EQCh. 1 - Prob. 74EQCh. 1 - Prob. 75EQCh. 1 - Heterolytic cleavage of the CO bond to yield a...Ch. 1 - Prob. 77EQCh. 1 - Prob. 78EQCh. 1 - Prob. 79EQCh. 1 - Prob. 80EQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- reaction scheme for C39H4202 Hydrogenation of Alkyne (Alkyne to Alkene) show reaction (drawing) pleasearrow_forwardGive detailed mechanism Solution with explanation needed. Don't give Ai generated solutionarrow_forwardShow work with explanation needed....don't give Ai generated solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Photochemistry : Introduction to Basic Theory of Photochemical Process [Part 1]; Author: Dr. Vikrant Palekar;https://www.youtube.com/watch?v=2NDOL11d6no;License: Standard YouTube License, CC-BY
Photochemistry-1; Author: CH-08:ARYABHATT [Mathematics, Physics, Chemistry];https://www.youtube.com/watch?v=DC4J0t1z3e8;License: Standard Youtube License