
(a) Consider a carbon atom in its ground state. Would such an atom offer a satisfactory model for the carbon of methane? If not, why not? (Hint: Consider whether a ground state carbon atom could be tetravalent, and consider the bond angles that would result if it were to combine with hydrogen atoms.)
(b) Consider a carbon atom in the excited state:
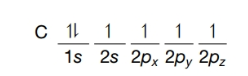
Excited state of a carbon atom
Would such an atom offer a satisfactory model for the carbon of methane? If not, why not?
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Living By Chemistry: First Edition Textbook
Biology: Life on Earth (11th Edition)
Fundamentals of Anatomy & Physiology (11th Edition)
Microbiology: An Introduction
Campbell Biology (11th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- a) Draw the octahedral mer-[FeCl3(CN)3] complex and determine its point group. Use proper wedges and dashes in order to illustrate 3 dimensional details. Use the point group to determine if the complex has a resulting net dipole moment and describe its allowed direction with respect to its symmetry elements (if applicable). ード M 4- b) Substitute one chlorido ligand in mer-[FeCl3(CN)3] 4 with one fluorido ligand. Determine all possible isomers and their corresponding point groups. Use the point groups to determine if the complexes have resulting net dipole moments and describe their allowed direction with respect to its symmetry elements (if applicable). The number of complex sketches below is not necessarily indicative of the number of isomers. 4- 4- ☐☐☐ c) Substitute two chlorido ligands in mer-[FeCl3 (CN)3] 4 with two fluorido ligands. Determine all possible isomers and their corresponding point groups.. Use the point groups to determine if the complexes have resulting net dipole…arrow_forwardShow work. don't give Ai generated solutionarrow_forwardDifferentiate electron spin and electron spin moment.arrow_forward
- Differentiate between nuclear spin and electron spin.arrow_forwardDraw the trigonal prismatic MH6 molecular compound, where M is a 3d transition metal. a) Draw the trigonal prismatic MH6 molecular compound and determine its point group. b) i. What is the symmetry species for the 4s orbital on the central metal? ii. What is the symmetry species for the 3dx²-y² orbital on the central metal? Note: The z-axis is the principal axis. iii. Suggest a crystal field energy diagram for a d² electron configuration in a trigonal prismatic coordination environment. Label the metal d-orbital with their corresponding symmetry species label. Use the appropriate character table in the resource section.arrow_forwardPlease correct answer and don't used hand raiting don't used Ai solutionarrow_forward
- Don't used Ai solutionarrow_forwardShow work. Don't give Ai generated solutionarrow_forwardthis is an organic chemistry question please answer accordindly!! please post the solution draw the figures on a paper please hand drawn and post, please answer EACH part till the end and dont just provide wordy explanations, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning


