
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 49P
Assign the missing formal charges.
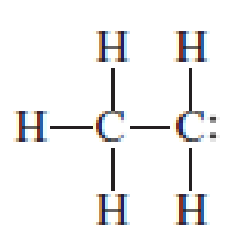
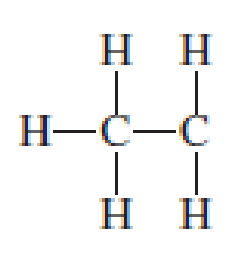
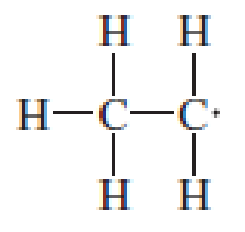
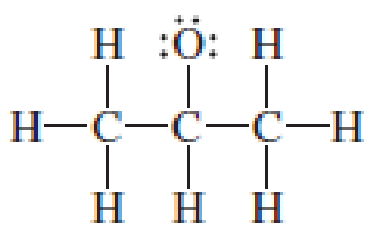
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The formal charge on N-1 is [Select]
The formal charge on N-2 is [Select]
The formal charge on N-3 is [Select]
The overall charge on the azide ion is
[Select]
A
Give typed explanation not written
Calculate the formal charge of oxygen in the compound
H.
none of the above
Chapter 1 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 1.1 - Oxygen has three isotopes, 16O, 17O, and 18O. The...Ch. 1.2 - Prob. 2PCh. 1.2 - How many valence electrons do chlorine, bromine,...Ch. 1.2 - Look at the relative positions of each pair of...Ch. 1.3 - a. Find potassium (K) in the periodic table and...Ch. 1.3 - Which bond is more polar?Ch. 1.3 - Which of the following has a. the most polar bond?...Ch. 1.3 - Use the symbols + and to show the direction of...Ch. 1.3 - After examining the potential maps for LiH, HF,...Ch. 1.4 - An atom with a formal charge does not necessarily...
Ch. 1.4 - Prob. 12PCh. 1.4 - a. Draw two Lewis structures for C2H6O. b. Draw...Ch. 1.4 - Draw the lone-pair electrons that are not shown in...Ch. 1.4 - Prob. 16PCh. 1.4 - Which of the atoms in the molecular models in...Ch. 1.4 - Prob. 18PCh. 1.7 - What orbitals are used to form the 10 sigma bonds...Ch. 1.9 - Put a number in each of the blanks: a. ___ s...Ch. 1.11 - Predict the approximate bond angles in a. the...Ch. 1.11 - According to the potential map for the ammonium...Ch. 1.12 - Prob. 25PCh. 1.13 - a. Predict the relative lengths and strengths of...Ch. 1.13 - Prob. 28PCh. 1.14 - Which of the bonds in a carbonoxygen double bond...Ch. 1.14 - Caffeine is a natural insecticide, found in the...Ch. 1.14 - a. What is the hybridization of each of the carbon...Ch. 1.14 - Prob. 33PCh. 1.14 - Describe the orbitals used in bonding and the bond...Ch. 1.15 - Account for the difference in the shape and color...Ch. 1.15 - Which of the following molecules would you expect...Ch. 1 - Draw a Lewis structure for each of the following...Ch. 1 - Prob. 38PCh. 1 - What is the hybridization of all the atoms (other...Ch. 1 - Prob. 40PCh. 1 - Draw the condensed structure of a compound that...Ch. 1 - Prob. 42PCh. 1 - Prob. 43PCh. 1 - Draw a Lewis structure for each of the following...Ch. 1 - Prob. 45PCh. 1 - List the bonds in order from most polar to least...Ch. 1 - What is the hybridization of the indicated atom in...Ch. 1 - Write the Kekul structure for each of the...Ch. 1 - Assign the missing formal charges.Ch. 1 - Predict the approximate bond angles for the...Ch. 1 - Prob. 51PCh. 1 - a. Which of the indicated bonds in each compound...Ch. 1 - In which orbitals are the lone pairs in nicotine?...Ch. 1 - Draw the missing lone-pair electrons and assign...Ch. 1 - Rank the following compounds from highest dipole...Ch. 1 - Prob. 56PCh. 1 - a. Which of the species have bond angles of 109.5?...Ch. 1 - Prob. 58PCh. 1 - Sodium methoxide (CH3ONa) has both ionic and...Ch. 1 - a. Why is a H 8 H bond (0.74 ) shorter than a C 8...Ch. 1 - Which compound has a larger dipole moment, CHCl3...Ch. 1 - Which compound has a longer C 8 Cl bond?Ch. 1 - Prob. 63PCh. 1 - The following compound has two isomers. One isomer...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following statements concerning the structures below is/are true? Formal charges are not shown. You can select more than one, or none of, these statements. H H- : 0: :Ö: :0: A :Ö: :0: D :O: H -Ö—H HÖ :0: :0: B :Ö: HÔ—s—ö—H :0: -Ö—H E H- :O: :Ö: :0: C The formal charge of S is zero in structure A. The formal charge of S is zero in structure E. On the basis of formal charges, structure A is the most important structure. The formal charge of S is +1 in structure C. In structure D, the formal harge is -1 for the O atom that has a double bond to S. Structure B is unrealistic because there are too many electrons around S. -Ö-Harrow_forwardAn incomplete structure of a porphyrin ring is shown below. The structure is missing three pi bonds and does not show the non-zero formal charges on two atoms. Complete the structure of the porphyrin ring with the missing pi bonds and include all of the formal charges..arrow_forwardHow to calculate formal charge for NCO- ?arrow_forward
- Determine the formal charges for each element in the perchlorate ion. Group of answer choicesarrow_forwardWrite in any formal charges not equal to zero. If there are none, please check the box below. :CEN: OThere are no missing formal charges.arrow_forwardThe Lewis structure for the chlorate ion is Calculate the formal charge on the chlorine (C1) atom. Express your answer as an integer. ► View Available Hint(s) formal charge on Cl = Submit Previous Answers Incorrect; Try Again; 3 attempts remaining Part B Calculate the formal charge on each of the oxygen (O) atoms labeled a, b, and c in the following Lewis structure. b :O: Express your answers as integers separated by commas. ► View Available Hint(s) b :0: formal charge on Oa, Ob, Oc c =arrow_forward
- Please don't provide hand written solution....arrow_forwardDraw three valid Lewis structures for the SiPSH (connected Si-P-S-H) that obey the octet rule and write any non-zero formal charges above the structures and circle the best structure.arrow_forwarda.)Draw a Lewis diagram for IO4- in which the central I atom has a formal charge of zero and show all NONZERO formal charges on all atoms. note overall charge of ion is -1 b.)Draw a Lewis structure for IO4- in which the octet rule is satisfied on all atoms and show all NONZERO formal charges on all atoms. C.Based on formal charge, what is the best Lewis structure for the ion? smallest formal charge or octet rule satisfied for all atomsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY