
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
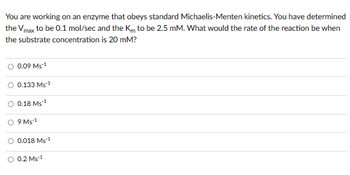
Transcribed Image Text:You are working on an enzyme that obeys standard Michaelis-Menten kinetics. You have determined
the Vmax to be 0.1 mol/sec and the Km to be 2.5 mM. What would the rate of the reaction be when
the substrate concentration is 20 mM?
0.09 MS-1
O 0.133 Ms-1
O 0.18 Ms ¹
9 Ms-1
O 0.018 Ms-1
0.2 MS-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- You begin to study enzyme Z, which catalyzes a simple reversible reaction that interconverts compound S and compound P. You observe that the ∆G´° for the S to P conversion to be –6 kJ/mol, and that compound S has ∆G´° for binding to enzyme Z of –15 kJ/mol, while compound P has a ∆G´° for binding to enzyme Z of –13 kJ/mol. Please explain the effect of enzyme Z on conversion of S to P. (Your answer should include a graph qualitatively showing energy versus reaction progress; however, you still need to explain youranswer in words!) not sure how to make the correct graph.arrow_forwardWhen reviewing a Michaelis-Menten Saturation Curve, at first the rate of the reaction is relatively constant, but the rate decreases as the substrate is used up and eventually reaches a plateau. Afte reaching this plateau, what would speed up the reaction again? Adding more substrate Adding heat Adding more enzyme Adding cofactorsarrow_forwardFor an enzyme-catalyzed reaction, the velocity was determined at two different concentrations of the substrate. Estimate the value of Vmax. [S] (MM) 10 20 88 nmol/min 79 nmol/min 67 nmol/min V(nmol/min) 51 nmol/min 27 Not enough information is given to form a reasonable estimation. 48arrow_forward
- The following data were collected in the study of a new enzyme and an inhibitor of the new enzyme: Vo (nmol/sec) [S] (uM) 1.3 - Inhibitor + Inhibitor 2.50 0.62 2.6 4.00 1.42 6.5 6.30 2.65 13.0 7.60 3.12 26.0 9.00 3.58 What is the Km of the inhibited enzyme reaction?arrow_forwardPlease don't provide handwritten solution ....arrow_forwarda particular enzyme catalyzes a single reactant S to a single product P, following michaelis-menten kinetics rp=(VmaxCs) / (Km + Cs) 1. A reaction with this enzyme is carried out at very low substrate concentrations. Draw and label a curve on the plot that describes the reaction kinetics under those conditions.arrow_forward
- Suppose you were running out of substrate (PNPP) and decided to reduce the concentration used in your reactions (25 µM, 50 µM, 100 µM, 200 µM, 300 µM) by a factor of 100. How might this affect your kinetic analysis?arrow_forwardAt what substrate concentration would an enzyme with a Km of 0.005M operate at one quarter of its Vmax? What would be the Km for an enzyme if it is operating at 90% of its Vmax when the substrate concentration is 0.01Marrow_forwardFor an enzyme kinetics experiment, a student prepared a reaction mixture by mixing 450 microliters of 0.75mM PNPP with 4.25ml of 0.2M Tris-HCl buffer. When he is ready to measure the absorbance, he added 0.3ml of Alkaline Phosphatase to the mixture and mixed thoroughly. What is the substrate concentration at the beginning of the reaction in mM ?arrow_forward
- The typical Michaelis-Menten equation mathematically describes the overall rate of the reaction as V (this is because biologists don't like math). What does V actually mean? (write the definition of V in differential equation form). V= d( )/dt Reaction rate Substrate concentration V max ·½V TURKarrow_forwardFrom a kinetics experiment, kcat was determined to be 295sec-1. For the kinetic assay, 0.3mL of a 0.25mg/mL solution of enzyme was used, and the enzyme has a molecular weight of 125,000 g/mole. Assume a reaction volume of 3mL. Calculate Vmax (µM∙min-1) for the enzyme and catalytic efficiency ( in M-1sec-1) for the enzyme. The Km for the enzyme was determined to be 2.55 x 10-2M.arrow_forwardYou run a series of assays at 25°C on enzyme A. You measure the velocity for a range of S concentrations. What is the Km (in mM) for Enzyme A?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON