
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Based on the chart below, comment on the effect of reaction time on the reaction.
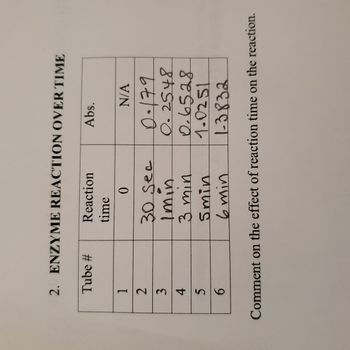
Transcribed Image Text:2. ENZYME REACTION OVER TIME
Tube #
1
2
3
4
5
6
Reaction
time
0
30 Sec
Imin
3 min
Smin
6 min
Abs.
N/A
0.179
0.2548
0.6528
1.0251
1-3832
Comment on the effect of reaction time on the reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- List the local effectors of enzyme kinetics and give an example for one of them.arrow_forwardEffects of Enzyme concentration on reaction rate. Question: prediction for the effect of enzyme concentration on reaction ratearrow_forwardPlease handraw this graph with all the necessary detailed information: Imagine that I text enzyme rate for four different temperatures: 10 degrees celsius, 20 degrees celsisus, 30 degree celsius, and 40 degree celsius, in separate tubes. The enzyme appears to work faster as temperature increases, but completely ceases activity at 40 degrees celcius. Sketch a graph to show this outcome, but here you will graph product formation (nmoles/mL) vs. time (minutes). The graph should be 4 lines and HANDDRAWN. Include a legend if necessary. You do not need precise quantitivate values, but most show the correct trends on the graph.arrow_forward
- An uncatalyzed reaction has keq=50. in the presence of an appropriate enzyme.the forward rate of the reaction increased by 20-fold.what is the equilibrium constant in the presence of the enzyme?arrow_forwardWhat Can We Learn About Biochemical Events From Thermodynamic Parameters?arrow_forwardDiscuss an enzyme that acts as a catalyst in a biological system. What reaction(s) does it catalyze? What kinds of problems arise if the enzyme isn't working properly? In what ways is the enzyme's activity regulated? Other interesting facts about the enzyme? Don't forget to cite your source(s).arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON