
World of Chemistry, 3rd edition
3rd Edition
ISBN: 9781133109655
Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher: Brooks / Cole / Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Please write out the answers as shown here please. It just helps to read it easier. Thank you :).

Transcribed Image Text:When the following equation is balanced properly under acidic
conditions, what are the coefficients of the species shown?
CIO4 +
Cd-
☐ CIO3 +
Cd2+
Water appears in the balanced equation as a
product, neither) with a coefficient of
(reactant,
(Enter 0 for neither.)
How many electrons are transferred in this reaction?
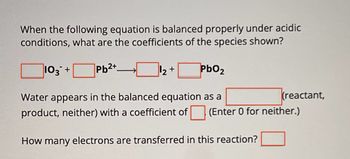
Transcribed Image Text:When the following equation is balanced properly under acidic
conditions, what are the coefficients of the species shown?
103 +
Pb2+
+
PbO2
Water appears in the balanced equation as a
(reactant,
product, neither) with a coefficient of (Enter 0 for neither.)
How many electrons are transferred in this reaction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- When the following equation is balanced properly under basic conditions, what are the coefficients of the species shown? S2032- + F2- So32- + Water appears in the balanced equation as a (reactant, product, neither) with a coefficient of (Enter 0 for neither.) How many electrons are transferred in this reaction?arrow_forwardS nomewUTK Answered Due Today, 11.3FM Fill in the Blanks Type your answers in all of the blanks and submit X, 2- s P F سه } AT T T & ce A €arrow_forwardConsider the energy diagrams (I, II, and III) for the three different reactions shown below when answering questions 18(a) – (f). Write NA if an applicable answer is not given.arrow_forward
- [Review Topics] [References] Use the References to access important values if needed for this question. When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? Cl2 + H3ASO4- HCIO + H,AsO3 Water appears in the balanced equation as a (reactant, product, neither) with a coefficient of (Enter 0 for neither.) How many electrons are transferred in this reaction? Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardWhen the following equation is balanced properly under basic conditions, what are the coefficients of the species shown?H2BO3- + Br-Br2 + BWater appears in the balanced equation as a fill in the blank 5 (reactant, product, neither) with a coefficient of . (Enter 0 for neither.)How many electrons are transferred in this reaction?arrow_forward2. For each reaction, predict the product(s), balance the equation, and note the reaction type. 1. Pb(NO3)2 + NazCO3 → 2. CAH10 + O2 - + 3. Zn + CuSO4 - + heat CaCO3 "S 5. Na + H20 - 6. Са + O2 - 7. MgSO, + LIOH - 8. Al + NİSO, 9. SO2 + H2O → heat Hgo 10. 4.arrow_forward
- When the following equation is balanced properly under basic conditions, what are the coefficients of the species shown? Br2 + 2- SO3 2- SO4 Br + Water appears in the balanced equation as a (reactant, product, neither) with a coefficient of (Enter 0 for neither.) How many electrons are transferred in this reaction?arrow_forwardHow do you know what power to raise the product and reactant to in the Nerst equation?arrow_forwardAnswer number 6 to 8 only pls. I don't want to waste my money here.arrow_forward
- Consider the plot of potential energy E vs. reaction progress for an exothermic chemical reaction. Which of the assignment is not correct? progress of reaction line A: E, of the forward reaction line D: E for the reverse reaction line B: AHF of the reactants line C: AHf of the products all are correct Moving to another question will save this response. B.arrow_forwardName:Tashina B. 4-16-22 Period: 7 Conservation of Mass - Balance Equations Skeleton ** Success Criteria: I can determine that in a chemical reaction matter just changes forms, but the amounts of matter do not change. Directions: Place the number on the blank line for the coefficient that makes the following reactions balanced. 1. NaClO3- NaCl + 0₂ 2. (NH4)3PO4 + Pb3(PO4) 4+ NH4NO3 3. ZnS + Al₂S3 Ag₂S → Sn(NO₂)4+ KBr + C₂H₁7 + KOH + 5. 6. 7. 8. Pb(NO3)4 → AIP → Ag + Pt3N4 → Fe(OH)₂ O₂ → Co3(PO4)2 → Zn₂P₂ + S8 Sn, N4 + KOH + CO₂ + H₂O K₂PO4 + Pt(NO₂)4 FeBr3 Co(OH)2arrow_forwardProvided is a picture of my problem. I understand that According to Le Chatelier's principle when stress is applied on reaction at equilibrium then the reaction will shift towards those direction where the stress is released. I also understand that the answer for a. Is move right, b. Is move right, and c. Is shift left, but I still don’t understand the knowledge behind why stress is released on this sides in those particular situations. Please help by providing stepsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
