
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
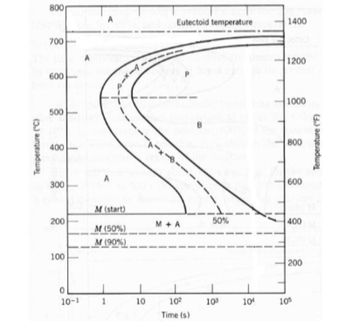
Please draw each scenario using the TTT diagram
Transcribed Image Text:Temperature (°C)
800
700
600
500
400
300
200
100
0
10-1
A
M (start)
M (50%)
M (90%)
1
1
10
Eutectoid temperature
M + A
I
10²
Time (s)
50%
10³
10⁰
1400
1200
1000
800
600
400
200
105
Temperature (°F)

Transcribed Image Text:time-temperature treatments. In each case assume that the specimen begins at 760°C (1400°F)
and that it has been held at this temperature long enough to have achieved a complete and
homogeneous austenitic structure.
(iv) Cool rapidly to 400°C (750°F), hold for 200 s, then quench to room temperature.
Rapidly cool to 575°C (1065°F), hold for 20 s, rapidly cool to 350°C (660°F), hold
for 100 s, then quench to room temperature.
(vi) Rapidly cool to 250°C (480°F), hold for 100 s, then quench to room temperature in
water. Reheat to 315°C (600°F) for 1 h and slowly cool to room temperature.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 100 atm 1 atm 0.118 atm 114 °C 184 °C 535 °C Temperature (not to scale) a.) Use the above generic phase diagram, clearly identify where you would find the following: Gas, Liquid, Solid, Triple Point, Draw arrows and label to indicate the six transitions (melting, freezing, sublimation, deposition, vaporization and condensation) b.) Based on the above phase diagram, what phase would you be in at a pressure of 50 atm and 300°C? c.) Based on the above phase diagram at what temperature in °C would vaporization occur under normal conditions? d.) If the pressure was 0.050 atm and 425°C, what phase would you be in? e.) If the pressure was 0.118 atm and temperature was 114°C, what phase(s) would you be in? f.) At approximately what temperature would the normal freezing point be in °C? g.) If the pressure was 0.105 atm and starting at a temperature of 32°C to 450°C what phase changes would occur (put in increasing temperature order). Pressure (not to scale)arrow_forwardI asked for problems 6 and 7 to be answered, but I did not get a properly structured answered as the example shows on problem number 1. Here is the link to the questions I already had answered, could you please rewrite the answer so its properly answered as the example shows (Problem 1)? https://www.bartleby.com/questions-and-answers/it-vivch-print-reading-for-industry-228-class-date-name-review-activity-112-for-each-local-note-or-c/cadc3f7b-2c2f-4471-842b-5a84bf505857arrow_forwardI need these three parts answered, if you are unable to answer all three parts please leave it for another tutor to answer, thank you.arrow_forward
- Draw the cooling curve for steam cooling from 115C to -5 Label each phase (solid, liquid, gas), and each phase change (ie: vaporization, condensation, melt, freeze, sublimation, deposition, etc) on your graph. Reminder: Temperature is on your Y-axis and heat is on your X-axis.arrow_forwardI want a step by step working out on how all these values were obtained and what table was usedarrow_forwardPlease show all your work no ai. I'm trying to truly understand.arrow_forward
- The figure shows a wooden model to reproduce a metal piece ofaluminum through the sand casting process.a) How much mass (g or kg) of aluminum do you need to melt?b) How much energy Q (Joules) is required? Assume an initial temperature of 50 ° C and a100% thermal efficiency.c) What dimensions will the final metal piece have? all is in cmarrow_forwardI was given this image for the purpose of studying for an upcoming exam. No additional info was given. Any suggestions on how to study for potential questions that could be asked about this image?arrow_forwardUsng the phase chan For each thezgments below answer the assigned questions. Luestions will center around change in temperature, kinetic energy, particle speed, bonds breaking or forming. -Alo, make use of the following vocabulary words as they apply for each of the segments below GAS, SOLID, LIQUID, MEILTING, VAPORIZATION, FREEZING, CONDENSATION B-C 1. Wha process is taking place during this segment? 2. L chermal energy being added or removed during this segment? 1. Explain why the temperatture is not chaging during this segment. s the procE6s during this segment andothermic.or expthermic? C-D 5. What is thr sate of matterfor this segment? 6. What is happening to the temperature? 7. Explain why the temperatiure ischanging, 8. L this change an endothermic or exothumie process/ 9. Dexribe the kinetic energy during this sepment 10. Dexribe the particle speed during this gment.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY