
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:←
Chrome File Edit View History Bookmarks Profiles Tab Window Help
X A ALEKS - Mae Martinez - Learn
→ C
=
Content
www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lvgWyWxWInmDn7WsVrRAXK6XnHkiRvH2tl8oejVt1a_5KUzFNROTku6p3nmyrVpHt0GBA3Zh6TWPM1GrO_6...
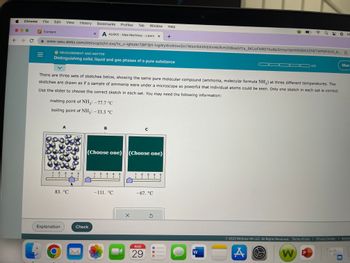
MEASUREMENT AND MATTER
Distinguishing solid, liquid and gas phases of a pure substance
melting point of NH3: -77.7 °C
boiling point of NH3: -33.3 °C
69
goo
There are three sets of sketches below, showing the same pure molecular compound (ammonia, molecular formula NH3) at three different temperatures. The
sketches are drawn as if a sample of ammonia were under a microscope so powerful that individual atoms could be seen. Only one sketch in each set is correct.
Use the slider to choose the correct sketch in each set. You may need the following information:
1 2
I
A
Explanation
D8
3
I
83. °C
8
00
4 5
Check
B
1
(Choose one) (Choose one)
2 3
1 I
-111. °℃
5
X
C
X +
2 3 4
1
I
-67. °C
AUG
29
Ś
W
A
a
0/5
W
00
2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center
P
M
Mae
Access
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution freezing point boiling point 3.4 g of ethylene glycol (C2H6O2) dissolved in 450. mL of water v (choose one) (choose one) 1(lowest) 3.4 g of sucrose (C12H22011) dissolved in 450. mL of water (choose one) 3 4(highest) (choose one) 3.4 g of sodium chloride (NaCI) dissolved in 450. mL of water (choose one) 450. mL of pure water (choose one) (choose one)arrow_forwardFour liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution 1.0 g of hydrochloric acid (HCI) dissolved in 200. mL of water 1.0 g of potassium hydroxide (KOH) dissolved in 200. mL of water 1.0 g of potassium sulfate (K₂SO4) dissolved in 200. mL of water 200. mL of pure water freezing point (choose one) O (choose one) (choose one) (choose one) O X boiling point (choose one) ✪ (choose one) O (choose one) O (choose one) O 5arrow_forwardFour liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution 8.8 g of sodium bromide (NaBr) dissolved in 500. mL of water 8.8 g of glucose (C6H₁206) dissolved in 500. mL of water 8.8 g of sodium chloride (NaCl) dissolved in 500. mL of water 500. mL of pure water freezing point (choose one) ✓ (choose one) ✓ (choose one) ✓ (choose one) ✓ X boiling point (choose one) ✓ (choose one) (choose one) (choose one) ✓arrow_forward
- There are 3 solids from different compounds: HF, FeCl3, and CsI (cesium iodide). These three solids are placed in 3 different containers, and are randomly labeled as A, B, and C. It is found that (1) the solid in container A has the highest melting point and (2) the solid in container B has the lowest melting point. Which of the following statements is correct about these solids? a. HF (s) in container A b. CsI (s) in container C. c. FeCl3 (s) in container C. d. FeCl3 (s) in container B.arrow_forwardEnter your answer in the provided box. The vapor pressure of ethanol is 1.00 × 102 mmHg at 34.90°C. What is its vapor pressure at 53.23°C? ( AHvap for ethanol is 39.3 kJ/mol.) mmHgarrow_forwardThe human body obtains 950 kJ of energy from a candy bar. If this energy were used to vaporize water at 100.0 ∘C, how much water (in liters) could be vaporized? The enthalpy of vaporization of water at 100.0 ∘C is 40.7 kJ⋅mol−1. (Assume the density of water is 1.00 g/mL.) Express your answer in liters to three significant figures.arrow_forward
- Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. ? Note: the density of water is 1.00 g/mL. solution 7.6 g of potassium iodide (KI) dissolved in 350. mL of water 7.6 g of propylene glycol (C3H8O₂) dissolved in 350. mL of water 7.6 g of hydroiodic acid (HI) dissolved in 350. mL of water 350. mL of pure water freezing point (choose one) î ✓ (choose one) 1(lowest) 2 3 4(highest) boiling point (choose one) (choose one) (choose one) (choose one) O 000 18 Ararrow_forwardFour liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select 'I' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select 'I' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL.arrow_forwardFour liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution 1.1 g of hydrobromic acid (HBr) dissolved in 350. mL of water 1.1 g of potassium chloride (KCI) dissolved in 350. mL of water 1.1 g of glycerin (C3H8O3) dissolved in 350. mL of water 350. mL of pure water freezing point (choose one) (choose one) (choose one) (choose one) ✓ X boiling point (choose one) (choose one) (choose one) (choose one) Sarrow_forward
- Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution 7.2 g of sodium chloride (NaCl) dissolved in 150. mL of water 7.2 g of propylene glycol (C3H₂O₂) dissolved in 150. mL of water 7.2 g of sucrose (C₁2H22011) dissolved in 150. mL of water 150. mL of pure water freezing point (choose one) (choose one) 1 (lowest) 2 3 4(highest) boiling point (choose one) ✓ (choose one) ✓ (choose one) ✓ (choose one)arrow_forwardFour liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select 'I' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL.arrow_forwardA 0.505 g sample of steam at 104.1 °C is condensed into a container with 5.88 g of water at 14.8 °C. What is the final temperature of the water mixture if no heat is lost? The specific heat of water is 4.18 J g⋅ °C, the specific heat of steam is 2.01 J g⋅ °C, and ΔHvap=−40.7 kJ/mol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY