
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points.
For example, select 'I' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on.
In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on.
Note: the density of water is 1.00 g/mL.
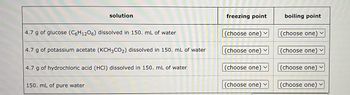
Transcribed Image Text:solution
4.7 g of glucose (C6H1206) dissolved in 150. mL of water
4.7 g of potassium acetate (KCH3CO₂) dissolved in 150. mL of water
4.7 g of hydrochloric acid (HCI) dissolved in 150. mL of water
150. mL of pure water
freezing point
(choose one)
(choose one)
(choose one) ✓
(choose one)
boiling point
(choose one)
(choose one)
(choose one) ✓
(choose one)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution freezing point boiling point (choose one) ↑ 6.7 g of potassium iodide (KI) dissolved in 350. mL of water (choose one) O (choose one) (choose one) ↑ 6.7 g of hydroiodic acid (HI) dissolved in 350. mL of water 6.7 g of sucrose (C₁2H22011) dissolved in 350. mL of water (choose one) (choose one) î 350. mL of pure water (choose one) ✪ (choose one) × Ś ?arrow_forwardREFER TO IMAGEarrow_forwardFour liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution 6.0 g of ethylene glycol (C₂H6O2) dissolved in 200. mL of water 6.0 g of potassium sulfate (K₂SO4) dissolved in 200. mL of water 6.0 g of sucrose (C12H22011) dissolved in 200. mL of water 200. mL of pure water freezing point ✓ (choose one) 1(lowest) 2 3 4(highest) (choose one) X boiling point (choose one) (choose one) (choose one) (choose one) Śarrow_forward
- This graph shows how the vapor pressure of three liquids varies with temperature: 900 800 700 600 500 400 300 200 octane acetylacetone orthoxylene 100. 100 110 120 130 140 temperature, °C Use the graph to answer the following questions: Which liquid is the most volatile? most volatile: choose one Which is the least volatile? least volatile: choose one O°c octane: What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be graded correct. acetylacetone: orthoxylene: Suppose a beaker of acetylacetone is put inside a sealed tank containing acetylacetone gas at 119. degree C and 206. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? more less the same vapor pressure, torrarrow_forwardFour liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution freezing point boiling point 7.3 g of glycerin (C3H8O3) dissolved in 100. mL of water (choose one) (choose one) 7.3 g of glucose (C6H1206) dissolved in 100. mL of water (choose one) (choose one) 7.3 g of potassium iodide (KI) dissolved in 100. mL of water (choose one) (choose one) 100. mL of pure water (choose one) (choose one)arrow_forwardShow the steps and reagents necessary to prepare the following compound from organic compounds having six carbons or less. Use retrosynthetic analysis as a tool to guide your synthesis. Br Brarrow_forward
- Enter your answer in the provided box. The vapor pressure of a liquid doubles when the temperature is raised from 75°C to 85°C. At what temperature will the vapor pressure be five times the value at 75°C?arrow_forwardThe organic compound trans-anethole is found in many oils/flavorings. This compound has a melting point of 50. ∘F. On Tuesday (this is a true story), a friend left a small vial of this compound in her back seat of her car outside for an hour and it solidified. At what temperature in ∘C does this compound freeze? We used this flavoring to make a holidy candy flavored with anise.arrow_forwardIn addition to filling in the blanks below, show all of your work for this problem on paper for later upload. The heat of vaporization of isopropanol is 44.0 kJ/mol. The vapor pressure of isopropanol at 400 torr is 67.8 °C. Calculate the normal boiling point of isopropanol. Enter your value in the first box and an appropriate unit of measure in the second box.arrow_forward
- Four liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution freezing point boiling point 2.0 g of ethylene glycol (C2H602) dissolved in 300. mL of water (choose one) (choose one) 2.0 g of potassium acetate (KCH3CO2) dissolved in 300. mL of water (choose one) (choose one) 2.0 g of glucose (C6H1206) dissolved in 300. mL of water (choose one) (choose one) 300. mL of pure water (choose one) (choose one)arrow_forwardCarbon tetrachloride, CCI4 , has a vapor pressure of 213 torr at 40.°C and 971 torr at 85°C. What is the normal boiling point of CCI4? Boiling point =arrow_forwardFour liquids are described in the table below. Use the second column of the table to explain the order of their freezing points, and the third column to explain the order of their boiling points. For example, select '1' in the second column next to the liquid with the lowest freezing point. Select '2' in the second column next to the liquid with the next higher freezing point, and so on. In the third column, select '1' next to the liquid with the lowest boiling point, '2' next to the liquid with the next higher boiling point, and so on. Note: the density of water is 1.00 g/mL. solution freezing point boiling point 6.4 g of glucose (C6H1206) dissolved in 300. mL of water (choose one) O (choose one) 6.4 g of potassium sulfate (K2SO4) dissolved in 300. mL of water (choose one) (choose one) O 6.4 g of sodium bromide (NaBr) dissolved in 300. mL of water (choose one) (choose one) O 300. mL of pure water (choose one) E (choose one) Earrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY