
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
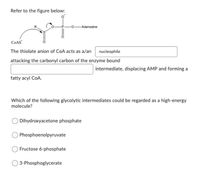
Transcribed Image Text:Refer to the figure below:
-Adenosine
COAS
The thiolate anion of CoA acts as a/an
nucleophile
attacking the carbonyl carbon of the enzyme bound
intermediate, displacing AMP and forming a
fatty acyl CoA.
Which of the following glycolytic intermediates could be regarded as a high-energy
molecule?
Dihydroxyacetone phosphate
Phosphoenolpyruvate
Fructose 6-phosphate
3-Phosphoglycerate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- During glycogen synthesis, glucose-1P is converted into a molecule called UDPG. This reaction also cleaves uridine triphosphate (UTP) forming uridine monophosphate and pyrophosphate (PPi). Provide four reasons why UTP can be used to power this reaction (no diagrams necessary).arrow_forwardOrder the cofactors based on their use in the mechanism of the a-etogluterate dehydrogenase complex. 1. Stabilizes a carbanion due to decarboxylation 2. Allows for the splitting of the carbon skeleton from the electron pair generated in a redox reaction 3. Enzyme bond electron carrier that is part of dihydrolipoyl dehydrogenase 4. Final electron acceptor in the overall reaction catalyzed by this complex 1 2 3 4 answer choices: lipoamide, biotin, 2 Fe - 2S cluster, TPP, NAD+, FADarrow_forwardFatty acids are activated for breakdown through the action of acyl-CoA synthestase. Which of the following statements regarding acyl-CoA synthetase is not true? ● It catalyzes the addition of CoA to the fatty acid One of the products of the reaction is ADP. O The free energy change for the reaction catalyzed by this reaction is close to 0 kJ/mol, but the subsequent hydrolysis of pyrophosphate drives the reaction forward. O The reaction results in the formation of a thioester bond.arrow_forward
- Explain why people with a hereditary deficiency of carnitine acyltransferase II have muscle weakness. Why are the symptoms more severe during fasting?arrow_forwardReferring to figure 5 and 8 which substrate molecule serves as the phosphate donor during substrate-level phosphorylation in step 10 of glycosis and the succinyl CoA-succinate step of the Krebs Cycle?arrow_forwardFor saturated fatty acids, these reactions can continue as you have drawn until all the carbons of the fatty acid have been oxidized to acetyl-CoA. What is problematic about an unsaturated fatty acid, such as C16:cis-9?arrow_forward
- Please answer this with the simplest answer , Please bolt the important answerarrow_forwardThe citric acid cycle is shown. The methyl carbon in acetyl CoA is labeled with C14C14 (shown in red). Identify which of the carbons in each intermediate will be labeled in the first round of the cycle by selecting the indicated carbon(s). Each question has multiple options, answering with only one option is incorrect. Which carbon(s) in α‑ketoglutarate will contain C14? 1 2 3 4 5 Which carbon(s) in succinyl‑CoA will contain C14? 1 2 3 4 Which carbon(s) in succinate will contain C14? 1 2 3 4 Which carbon(s) in fumarate will contain C14? 1 2 3 4 Which carbon(s) in malate will contain C14? 1 2 3 4 Which carbon(s) in oxaloacetate will contain C14? 1 2 3 4arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON