
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Look at the synthesis of citryl CoA by citrate synthase in the figure. Which of the 4 general catalytic mechanisms are present?
Catalysis by approximation
Covalent Catalysis
General Acid-Base Catalysis
Metal Ion Catalysis
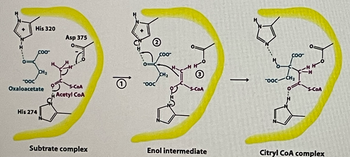
Transcribed Image Text:**Transcription for Educational Website:**
### Diagram Explanation: Enzymatic Reaction Pathway
The diagram illustrates the enzymatic conversion process involving oxaloacetate and acetyl CoA to form a citryl CoA complex through an enol intermediate. The process is shown in three main stages, each represented by a molecular structure within an active site, highlighted in yellow.
#### 1. Substrate Complex
- **Components**: Oxaloacetate and Acetyl CoA
- **Key Interactions**:
- **Histidine 320** and **Aspartate 375**: These amino acid residues are positioned to facilitate the interaction.
- **Histidine 274**: Interacts with the substrate complex, contributing to stabilization.
- **Macromolecules**:
- **Oxaloacetate**: Presented with carboxylate groups in blue.
- **Acetyl CoA**: Highlighted in magenta.
#### 2. Enol Intermediate
- **Description**: Transition state between substrates and product.
- **Key Reactions**:
- Step **①** and **②** denote shifts in molecular structure aiding the progression.
- **③** indicates the formation of the enol intermediate through enzyme-assisted protonations and deprotonations.
#### 3. Citryl CoA Complex
- **Resulting Product**: Formation of Citryl CoA.
- **Structure**:
- Combines previous molecular structures with key interactions via the amino acids.
- **Final Arrangement**: Stabilized by the active site residues, indicating successful transformation from reactants to a complex product.
The diagram provides a step-by-step visual understanding of the enzyme-catalyzed reaction, emphasizing the role of specific amino acids and the structural changes leading to product formation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Which of the following statements is correct for the reaction catalyzed by chymotrypsin? ○ The substrate carbon atom bonded to Ser195 in the acyl enzyme intermediate has a tetrahedral geometry ○ The Ser195 hydroxyl oxygen atom is the catalytic nucleophile for the deacylation phase of the overall reaction mechanism ○ The oxyanion hole accelerates the reaction by tightly binding the substrate carbonyl oxygen at the peptide bond that is cleaved ○ Asp102 stabilizes the tautomeric state of the catalytic histidine ○ The first reaction product contains a proton derived from an active-site water moleculearrow_forwardIf you can clearly visualize the chymotrypsin mechanism of action, you should be able to picture the structure of the transition state right after the enzyme attacks the first substrate. Think hard about what we have covered, and visualize that transition state accurately:arrow_forwardWhich of the following statements regarding enzymes and transition states is true? stabilization of the transition state must be less than stabilization of ES for catalysis to occur binding of substrate to an enzyme often causes strain, thus promoting transition state formation the transition state conformation of an enzyme catalyzed reaction is identical to the conformation seen in the uncatalyzed transition state formation of the transition state always assures that the reaction will proceed to product none of the above are truearrow_forward
- The structure of a metalloenzyme active site is down below(black picture). Describe, from a chemical and structural perspective, how the reactive site is designed to facilitate its catalytic reaction. The example below suggests the level of detail that is required. Make sure that you explain what the metal is doing, what the reaction is, and its biological significance.arrow_forwardCH₂OH 애 H2COPO 2- ATP OH 1 애 애 어 애 The class of the enzyme catalyzing the reaction shown in this figure is a(n) Choose the one best answer. ligase 매 +ADP 매arrow_forwardThe enzyme pyruvate carboxylase is discovered in a bacterium that was thought not to contain it; in this case study, you’ll see how researchers study and characterize the enzyme, and, ultimately, show how it fits into a metabolic pathway. Following purification of the PYC enzyme by avidin-Sepharose affinity chromatography, the investigators carried out several experiments to characterize the enzyme. First, they ran samples of the enzyme on denaturing and non-denaturing gels; the results are shown in the figure to the right. In addition, they ran the protein through a calibrated gel filtration column, the results of which indicated that the PYC enzyme had a molecular weight of 540 kiloDaltons. In the figure, the rightmost column are where molecular weight standards of various sizes would occur if they’d been on the gels. The catalytic properties of the PYC enzyme were assessed following purification. The activity of the enzyme was assayed in the presence of ATP, pyruvate, bicarbonate…arrow_forward
- You are trying to confirm that an enzyme you are studying has the following amino acid in its active site, and that this amino is critical for catalysis: HN- H3NⓇ Which of the following inhibitors could you use to provide evidence of the importance of this amino acid for enzyme function? Tetranitromethane 2,3-Butanedione Diethylpyrocarbonate lodoacetate Nevirapinearrow_forwardPlease don't provide hand written solution....arrow_forwardLysozyme catalyzes a "bi-bi" reaction, which means there are (how many) reactants and (how many) products. List, in order, the reactants that bind and the products that are released during a lysozyme-catalyzed reaction cycle -- be succinct but be specific. 1. First reactant = 2. First product = 3. Second reactant = 4. Second product %3Darrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON