
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
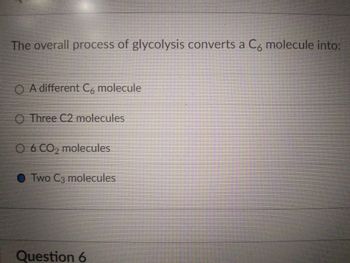
Transcribed Image Text:The overall process of glycolysis converts a C6 molecule into:
O A different C6 molecule
O Three C2 molecules
O 6 CO₂ molecules
Two C3 molecules
Question 6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Which of the following regarding the ATP Synthase is INCORRECT? Protons flow through the a and c subunits The translocation of four protons through the ATP synthase fuels the synthesis of one ATP molecule The F1 domain can function as an ATPase The y subunit rotates while the xß dimers are stationaryarrow_forwardATP synthesis in mitochondria requires all of the following EXCEPT (select two answers) a hydrogen ion concentration gradient across the inner mitochondrial membrane the oxidation of dinucleotide molecules that act as electron carriers fermentation of pyruvate into lactate to regenerate NAD+ the reduction O2, forming H2O the breakdown of glucosearrow_forwardWhich of the following statements about the aerobic respiration of a molecule of glucose is FALSE? O It requires pathways and membrane complexes in both the cytosol and mitochondria in eukaryotes. O 34 molecules of ATP are generated by oxidative phosphorylation. O It requires pathways and membrane complexes in the cytosol and plasma membrane in prokaryotes. O It can generate 38 molecules of ATP in prokaryotes. O 12 molecules of ATP are generated by substrate-level phosphorylation.arrow_forward
- Below is an Image of mitochondria, identify which numbered area respond to outer membrane, inner membrane, intermbrane and matrix described where the process of glycosis, pyruvates oxidation, citric acid cycle and electron transport occur.arrow_forward1,3-bisphosphoglycerate is used to produce ATP. Which of the two phosphates of 1,3- bisphosphoglycerate is transferred to ADP to make ATP. Explain why it is this specific phosphate and not the other one.arrow_forwardWhich of the following conditions will produce active glycogen phosphorylase? AMP АТР Phosphorylation of Serine 14 Glucose Glucose-6-Phosphate Phosphorylation of Serine 14 and AMP Phosphorylation of Serine 14 and ATParrow_forward
- Phosphorolysis is an important part of how our muscle and liver cells break down glycogen. What is phosphorolysis and what would be the consequence to the cell if hydrolysis was used as the mechanism for glycogen breakdown instead of phosphorolysis? We also learned about something very analogous to phosphorolysis called thiolysis. Where did we see thiolysis and what would be the consequence if hydrolysis was used for that reaction instead of thiolysis?arrow_forward44 45 46 47 48 50 27 Use the following figure to answer the question. CO2 Охаlоасеtic acid Phosphoenol- pyruvate AMP+ P K NADPH Cell I NADP+ ATP Malic acid Pyruvic acid Pyruvic acid Malic acid RUBP NADP CO2 Cell II NADPH Triose Phospho- glycerate Which of the following statements is true concerning the accompanying figure? O It represents a C4 photosynthetic system. It represents an adaptation that maximizes photorespiration. It represents a CAM photosynthetic system. It represents a C3 photosynthetic system. A Moving to another question will save this response. esc DII D 身 49arrow_forwardDuring cellular respiration, which of the following diffuses through ATP synthase? O Phosphates O Protons (H* ions) O ATP O Electrons O Carbon dioxide (CO2)arrow_forward
- Which chemical equation represents the breakdown of organic carbon (into inorganic form) thattakes place during glycolysis, the Krebs cycle, and electron transport (aerobic respiration)? A. C6H12O6 + C6H12O6 C12H22O11 + H2OB. 6CO2 + 12H2O C6H12O6 + 6O2 + 6H2OC. C12H22O11 + H2O C6H12O6 + C6H12O6D. C6H12O6 + 6O2 + 6H2O 6CO2 + 12H2OE. all of the abovearrow_forwardAda is a pregnant 28 year old vegan. Her pre-pregnancy weight was 132 Ibs (60 kg), and her BMI was 24. She is now 30 weeks along, and feeling fine. Her current weight is 153 Ibs (68.5 kg).arrow_forwardLoss of this enzyme would be lethal to the cell CH₂-CH-CH₂ OH OH Glycerol- phosphate Loss of this enzyme would severely reduce the exchange of lipids between the cytosol and lumen P CH₂-CH-CH₂ O O I =C C=O Loss of this enzyme would lead to membrane phospholipids with identical phosphate head groups Phosphatase Acyl transferase Loss of this enzyme would result in membranes deficient in phosphatidylcholine Choline head Cytosol group ER lumen Choline phosphotransferase Loss of these enzymes would prevent lipid transfer between the ER and mitochondria Flippase lipid exchange proteinsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON