
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
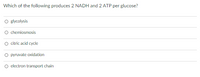
Transcribed Image Text:Which of the following produces 2 NADH and 2 ATP per glucose?
O glycolysis
chemiosmosis
citric acid cycle
O pyruvate oxidation
O electron transport chain
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following is(are) consumed in the process of converting glycerol to dihydroxyacetone phosphate? O both a and b O neither a nor b O NAD* O ATP Ne « Previousarrow_forwardChoose all of the molecules that are made in acetyl-CoA formation and the citric acid cycle that are important to note. NADH FADH2 CO2 ATP pyruvate glucosearrow_forwardDuring aerobic respiration, H20 is formed. Where does the O atom come from? pyruvate 02 glucose CO2arrow_forward
- Which enzyme uses ATP to phosphorylate a substrate during glycolysis? - Phosphoglycerokinase - Phosphofructokinase 1 - Pyruvate kinase - Hexokinase - Glyceraldehyde-3-phosphate dehydrogenasearrow_forwardThroughout the electron transport chain many reactions occur. What happens to NADH eventually? NADH is reduced NADH is not used in ETC. ONADH remains unchanged in ETC but is used later. ONADH is oxidizedarrow_forwardWhich one of the following best describes the electron transport chain? Electrons are passed from one carrier to another releasing a little energy at each step Electrons are pumped across a membrane by active transport Glucose is broken down to a three-carbon compound in preparation for the citric acid cycle Hydrogen atoms are added to CO2 to make an energy-rich compound Acetyl CoA is fully oxidized to CO2arrow_forward
- During glycolysis step 7, ATP synthesis occurs through ______. The energy for this reaction comes _____ oxidative phosphorylation; indirectly from oxidation of NADH/FADH2 Gaining of electron; Hydrolysis of ADP Reduction of Oxygen; Dissipation of proton gradient substrate level phosphorylation; coupling directly to an exergonic reaction.arrow_forwardA cell is grown on minimal media containing only malic acid as a source of carbon and energy. What is the most likely place this compound would enter the metabolic cycle? Glycolysis Conversion of Pyruvate to Acetyl-CoA The TCA Cycle The Electron Transport Chainarrow_forwardWhich of the below cellular processes requires input of ATP energy? Group of answer choices ATP-synthase creating ATP Na+-K+-ATPase (sodium-potassium pump) activity Simple diffusion Pyruvate Oxidation The Electron Transport Chainarrow_forward
- Muscle tissues make lactate from pyruvate in order to do which of the following? Speed up the rate of glycolysis Get rid of pyruvate produced by glycolysis Regenerate nad+ Utilize the energy in pyruvate Produce additional co2arrow_forwardHow many ATP can be made from one molecule of thefollowing? Explain how this is done. Phosphoenolpyruvate NADH GTParrow_forwardThe normal flow of electrons through the electron transport chain is blocked by sodium azide. Which of the following would you predict to occur upon addition of sodium azide to a cell? Halt in pyruvate oxidation and continuation in ATP synthesis Continuation in pyruvate oxidation and halt in ATP synthesis Halt in both pyruvate oxidation and ATP synthesis Continuation in both pyruvate oxidation and ATP synthesisarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON