
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
The Krebs cycle reaction shown below is catalyzed by __ enzyme and ___ pays for this reaction note only major metabolites are shown
a. Synthetase, hydrolysis of acetyl CoA
b. Synthase, hydrolysis of acetyl CoA
c. Synthase, hydrolysis of NADH
d. Synthetase, hydrolysis of ATP
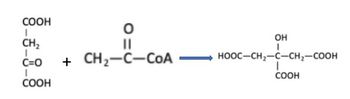
Transcribed Image Text:COOH
CH₂
I
C=O
I
COOH
O
||
+ CH₂-C-CoA
OH
HOOC–CH,—C—CH,—COOH
I
COOH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Can you please solve the following question, both parts please.arrow_forwardEnzyme 1 is Aconitase and the starting material goes through both the hydration and dehydration mechanism. I just need help with enzyme 2 and intermediate B, thank youarrow_forwardThe following reaction takes place during the synthesis of glycogen: Glucose 1-phosphate + UTP + H20 UDP-Glucose + 2Pi This reaction may be best described as a: a. exergonic, catabolic reaction O b. exergonic, coupled reaction C. oxidation-reduction reaction d. endergonic, substrate-level phosphorylation reaction e. exergonic, substrate-level phosphorylation reactionarrow_forward
- I've stated that acetyl-CoA is perhaps the most central compound to our metabolic processes. Explain why I would make such a bold statement in the context of the metabolic processes we've covered so far.arrow_forwardFor each step of the citric acid cycle, name the enzyme responsible for the chemical transformation that occurs and classify the enzyme type: Step 1. Step 2. Step 3. Step 4. Step 5. Step 6. Step 7. Step 8. i. oxidoreductase (oxidases, reductases, dehydrogenases) ii. transferase (transaminases, kinses) iii. hydrolase (lipases, proteases, nucleases, carbohydrases, phosphateses) iv. lyase (dehydratase, decarboxylase, deaminase, hydratase) v. isomerase (racemases, mutases) vi. ligase (synthetases, carboxylaces) Enzyme Name Enzyme Typearrow_forwardWhich of the following molecules shown are the reduced products produced in the citric acid cycle whose reduction directly helped drive oxidation? Select all that apply. Ensure you are selecting the product forms of the molecule, which here means the reduced form, and are only considering redox reactions! Do not include the pyruvate processing steps -- just the citric acid cycle itself. A. GDP B. NADH C. FAD D. Acetyl-CoA E. NAD+ F. CoA G. FADH2 H. GTP I. QH2 J. Qarrow_forward
- You are a genetic analyst and you have a patient with inherited defects of glycolysis. Specifically, this patient has severe symptoms that stem from issues in their erythrocytes (red blood cells). You find that your patient has a deficiency in Hexokinase (HK). Given this information and keeping in mind what you learned about the steps of glycolysis, bisphosphoglycerate (BPG), and the importance of 2,3 BPG for hemoglobin oxygen binding what do you predict will be the effect of this mutation? Select all that apply. decreased 2,3BPG concentrations reduced flow of metabolites through glycolysis increased 2,3BPG concentrations increased flow of metabolites through glycolysis decreased hemoglobin oxygen affinity increased hemoglobin oxygen affinity 000000arrow_forwardThe structure of a metalloenzyme active site is down below(black picture). Describe, from a chemical and structural perspective, how the reactive site is designed to facilitate its catalytic reaction. The example below suggests the level of detail that is required. Make sure that you explain what the metal is doing, what the reaction is, and its biological significance.arrow_forward7) The following questions apply to glycolysis (figure 3-41 on page 79 of the 12th edition), the Krebs cycle (figure 3-44 on page 82) and to oxidative phosphorylation (figure 3-45 on page 84). Calculate the total amount of ATP produced from one glucose molecule if: a. The oxidation of NADH + H* to NAD* + 2H* produced 4 ATP. b. The oxidation of FADH₂ to FAD + 2H* produced 1 ATP. c. Each step of the Krebs cycle that produces NADH+H* produced FADH2 instead. d. Each step of the Krebs cycle that produces FADH2 produced NADH + H+ instead. e. Sucrose is substituted for glucose.arrow_forward
- Can you please help me answer the following questions. An enzymatic pathway is organized so that the final product will act as a competitive inhibitor to the first enzyme in the pathway. This is an example of: A. denaturation of a substrate enzyme complex B. positive feedback inhibition C. negative feedback inhibition D. competive binding of a productarrow_forwardGAP + Pi + NAD+ ⟹1,3-BPG + NADH (Reaction 1) 1,3-BPG + ADP ⟹ 3-PG + ATP (Reaction 2) Which of the following statements concerning the information above is true? Select all that apply. A.Reaction 2 is an example of substrate level phosphorylation B.These reactions are written in a direction that would indicate gluconeogenesis is occurring in the liver C.The linking of Reaction 1 and Reaction 2 by the intermediate 1,3-BPG is an example of coupling of reactions D.Reaction 1 shows a redox reaction where the carbon skeleton is oxidized to generate electrons for a soluble electron carrier E.These reactions are irreversible under cellular conditionsarrow_forwardThe following reaction, the last reaction in beta-oxidation, is an example of a: Enzyme Enzyme-H S-CoA S-COA H2C H3C CoA s–CoA C H2 CH2 R S-COA S-COA S-COA O A. Nucleophilic acyl substitution O B. Electrophilic addition OC. Nucleophilic addition D. Aldol condensation O E. Hydrohalogenationarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON