
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
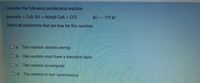
Transcribed Image Text:Consider the following biochemical reaction.
pyruvate + CoA-SH Acetyl-CoA + CO2
AG = -175 kJ
Select all statements that are true for this reaction.
a. This reaction absorbs energy
ఉ డ
Ob. This reaction must have a transition state
Oc. This reaction is exergonic
Od. This reaction is non-spontaneous
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 25. The AG" values for the two reactions are given. 1. 2. oxaloacetate + acetyl-CoA + H₂O → citrate + COASH oxaloacetate + acetate → citrate Enzymes for reactions 1 and 2 are citrate synthase and citrate lyase, respectively. Determine the AG" for the hydrolysis of acetyl-CoA acetyl-CoA + H₂O acetate + COASH + H+ plane een isoimorfbold bns (94) opg Vp1903 9911 AG¹⁰ ? AGO = -32.2 kJ/mol AG"=-1.9 kJ/molarrow_forwardFor the following enzymes (3-6) predict how the conditions will most likely affect the enzymes activity with one of the following and provide a 1 sentence explanation: a. increase activity b. decrease activity c. not likely to alter activity 3) alpha-ketoglutarate dehydrogenase complex binding to AMP when [AMP] is high. 4) phosphohexose isomerase binding NADH when [NADH] is high 5) phosphofructose-1 binding NAD+ when [NAD+] is high 6) pyruvate dehydrogenase complex binding to ATP when [ATP] is higharrow_forwardThe oxidation of Gly-3-P to 1,3-bisphosphoglycerate proceeds with an unfavorable AG which equals 6.3 KJ/mol, yet the flow through this point in the glycolytic pathway proceeds smoothly. Why???arrow_forward
- The following two reactions are coupled in the last step of glycolysis (catalyzed by the enzyme pyruvate kinase). РЕР + Н.О € Руr + Pi ADP + Pi → ATP + H2O AG° = -14.8 kcal/mol AG° = +7.3 kcal/mol D) The steady-state concentrations of ATP, ADP, and pyruvate in human erythrocytes are 2.24 mM, 0.25 mM, and 0.051 mM, respectively. What would be the concentration of PEP in these cells if the coupled reaction was at equilibrium?arrow_forwardGAP + Pi + NAD+ ⟹1,3-BPG + NADH (Reaction 1) 1,3-BPG + ADP ⟹ 3-PG + ATP (Reaction 2) Which of the following statements concerning the information above is true? Select all that apply. A.Reaction 2 is an example of substrate level phosphorylation B.These reactions are written in a direction that would indicate gluconeogenesis is occurring in the liver C.The linking of Reaction 1 and Reaction 2 by the intermediate 1,3-BPG is an example of coupling of reactions D.Reaction 1 shows a redox reaction where the carbon skeleton is oxidized to generate electrons for a soluble electron carrier E.These reactions are irreversible under cellular conditionsarrow_forward9. The equation below depicts the first step of the citric acid cycle. H0 CoA-SH CH-C + 0=C-CO0 HO C-COO citrate s-COA ČH2-COO synthase ČH2 -CO0 Citrate Acetyl-CoA Oxaloacetate AG" = -32.2 kJ/mol a) Explain the chemical conversions that take place during this step. b) Why is this reaction energetically favorable? Explain.arrow_forward
- The following reaction, the last reaction in beta-oxidation, is an example of a: Enzyme Enzyme-H S-CoA S-COA H2C H3C CoA s–CoA C H2 CH2 R S-COA S-COA S-COA O A. Nucleophilic acyl substitution O B. Electrophilic addition OC. Nucleophilic addition D. Aldol condensation O E. Hydrohalogenationarrow_forwardThe following incorrect description of the HMS path is () A, 6-P-glucose can be converted into pentose phosphate through this pathway B. Four-carbon and seven-carbon sugars can be provided through this route C. For every mole of carbon dioxide produced when 6-P-glucose is converted to pentose phosphate, it also produces 1 mole of NADPH D, 6-P-glucose is decomposed in this way without consumption of ATP Among the following enzyme-catalyzed reactions that can generate substrate level phosphorylation to generate GTP are () A, hexokinase B, enolase C, succinate thiokinase D, succinate dehydrogenase The limiting factor of fatty acid synthesis in cell fluid is (). A, condensation enzyme B, hydration enzyme C, lipoacyl group transferase D, acetyl-CoA carboxylase Which of the following amino acids is an essential amino acid? () A.Thr B.Lys C.Met D.Argarrow_forwardProvide a reasonable step-wise mechanism for the reaction below, involving TPP as acoenzyme (can abbreviate the pyrophosphate as R), and invoking any general acids or base fromacetolactate synthase that you might need .arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON