
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
a) Give the pKa
b) Give the Ka
c) Calculate the molar mass of the unknown. The concentration of added solution was 0.0936 M
d) Give the pH at the equivalence point
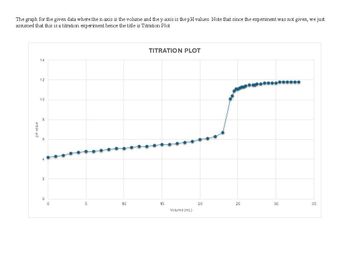
Transcribed Image Text:The graph for the given data where the x-axis is the volume and the y-axis is the pH values. Note that since the experiment was not given, we just
assumed that this is a titration experiment hence the title is Titration Plot
pH value
2
4
6
14
12
10
8
TITRATION PLOT
0
0
5
10
15
20
25
30
35
Volume (mL)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Which of the following acids and its conjugate base would you use to make a buffer with a pH of 3.00? Explain your reasons: formic acid, lactic acid, nitrous acid.arrow_forwardAnother way to treat data from a pH titration is to graph the absolute value of the change in pH per change in milliliters added versus milliliters added (pH/mL versus mL added). Make this graph using your results from Exercise 61. What advantage might this method have over the traditional method for treating titration data?arrow_forwardA 25.00-mL sample of gastric juice is titrated with a 0.0210MNaOH solution. The titration to the equivalence point requires 26.4mL of NaOH solution. If the equation for the reaction is HCl(aq)+NaOH(aq)NaCl(aq)+H2O(l) what is the molarity of HCl in the gastric juice?arrow_forward
- 7. Describe a buffered solution. Give three examples of buffered solutions. For each of your examples, write equations and explain how the components of the buffered solution consume added strong acids or bases. Why is buffering of solutions in biological systems so important?arrow_forwardExplain why the hydrolysis of salts makes it necessary to have available in a laboratory more than one acid-base indicator for use in titrations.arrow_forwardA substance that functions to prevent rapid, drastic changes in the pH of a body fluid by changing strong acids and bases into weak acids and bases is called an: a.salt. b.buffer. c.enzyme. d.coenzyme.arrow_forward
- Sulfanilic acid (NH2C6H4SO3H) is used in manufacturing dyes. It ionizes in water according to the equilibrium equation NH2C6H4SO3H(aq)+H2O(l)NH2C6H4SO3(aq)+H3O+(aq)Ka=5.9104 A buffer is prepared by dissolving 0.20 mol of sulfanilicacid and 0.13 mol of sodium sulfanilate (NaNH2C6H4SO3) in water and diluting to 1.00 L. Compute the pH of the solution. Suppose 0.040 mol of HCl is added to the buffer.Calculate the pH of the solution that results.arrow_forwarda.Calculate the pH of a buffer that is 0.1M in lactic acid, C2H4(OH)COOH, and 0.1M in sodium lactate, C2H4(OH)COONa. b.What is the pH of a buffer that is 1M in lactic acid and 1M in sodium lactate? c.What is the difference between the buffers described in parts a and b?arrow_forwardPredict the relative pH greater than 7, less than 7, etc. for water solutions of the following salts. Table 9.9 may be useful. For each solution in which the pH is greater or less than 7, explain why and write a net ionic equation to justify your answer. a.Sodium hypochlorite, NaOCl HOCl is a weak acid b.Sodium formate, NaCHO2 c.Potassium nitrate, KNO3 d.Sodium phosphate, Na3PO4arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning