
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
1) Write the reduction half reactions and find the reduction potential for each pair.
a. Zn/Zn2+
b. Cu/Cu2+
c. Al/Al3+
d. Ag/Ag1+
a. Zn/Zn2+
b. Cu/Cu2+
c. Al/Al3+
d. Ag/Ag1+
2) For each of the following voltaic cells, identify the anode, cathode, write the standard cell notation/diagram, and predict the cell potential.
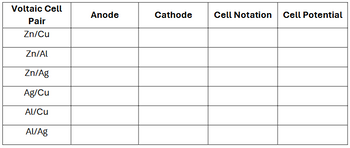
Transcribed Image Text:Voltaic Cell
Pair
Zn/Cu
Zn/Al
Zn/Ag
Ag/Cu
Al/Cu
Al/Ag
Anode
Cathode
Cell Notation Cell Potential
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Calculate the standard cell potential of the following cell at 25C. Sn(s)Sn2+(aq)I2(aq)I(aq)arrow_forwardAn electrolysis experiment is performed to determine the value of the Faraday constant (number of coulombs per mole of electrons). In this experiment, 28.8 g of gold is plated out from a AuCN solution by running an electrolytic cell for two hours with a current of 2.00 A. What is the experimental value obtained for the Faraday Constant?arrow_forwardGiven this reaction, its standard potential, and the standard half-cell potential of 0.34 V for the Cu2+ |Cu half-cell, calculate E° for the Fe(s)|Fe2+(aq) half-cell.arrow_forward
- The standard potential of the cell reaction Ag+(aq)+Eu2+(aq)Ag(s)+Eu3+(aq) is E = +1.23 V. Use the tabulated standard potential of the silver half-reaction to find the standard reduction potential for the europium half-reaction.arrow_forwardFor each reaction listed, determine its standard cell potential at 25 C and whether the reaction is spontaneous at standard conditions. (a) Mn(s)+Ni2+(aq)Mn2+(aq)+Ni(s) (b) 3Cu2+(aq)+2Al(s)2Al3+(aq)+3Cu(s) (c) Na(s)+LiNO3(aq)NaNO3(aq)+Li(s) (d) Ca(NO3)2(aq)+Ba(s)Ba(NO3)2(aq)+Ca(s)arrow_forwardA standard galvanic cell is constructed so that the overall cell reaction is 2A13++(aq)+3M(s)3M2+(aq)+2A1(s) Where M is an unknown metal. If G = 411 kJ for the overall cell reaction, identify the metal used to construct the standard cell.arrow_forward
- Consider a galvanic cell based on the following half-reactions: a. What is the standard potential for this cell? b. A nonstandard cell is set up at 25C with [Mg2+] = 1.00 105 M. The cell potential is observed to be 4.01 V. Calculate [Au3+] in this cell.arrow_forwardDetermine the overall reaction and its standard cell potential at 25 C for this reaction. Is the reaction spontaneous at standard conditions? Cu(s)|Cu2+(aq)Au3+(aq)|Au(s)arrow_forwardFor each of the reactions, calculate E from the table of standard potentials, and state whether the reaction is spontaneous as written or spontaneous in the reverse direction under standard conditions. (a) Cu2+(aq)+Ni(s)Cu(s)+Ni2+(aq) (b) 2Ag(s)+Cl2(g)2AgCl(s) (c) Cl2(g)+2I(aq)2Cl(aq)+I2(s)arrow_forward
- At 298 K, the solubility product constant for solid Ba(IO3)2 is 1.5 109. Use the standard reduction potential of Ba2+(aq) to find the standard potential for the half-reaction Ba(IO3)2(s)+2eBa(s)+2IO3(aq)arrow_forwardA galvanic cell is constructed in which the overall reactionis Cr2O72(aq)+14H2O+(aq)+6I(aq)2Cr3+(aq)+3I2(s)+21H2O(l) Calculate E for this cell. At pH 0, with [Cr2O72]=1.5M and [I]=0.40M, the cell potential is found to equal 0.87 V. Calculatethe concentration of Cr3+(aq) in the cell.arrow_forwardIt took 150. s for a current of 1.25 A to plate out 0.109 g of a metal from a solution containing its cations. Show that it is not possible for the cations to have a charge of 1+.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole