
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
None
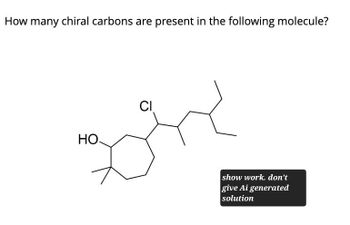
Transcribed Image Text:How many chiral carbons are present in the following molecule?
HO
CI
од
show work. don't
give Ai generated
solution
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Estradiol is a female hormone with the following structure: How many chiral carbon atoms are in estradiol?arrow_forwardDraw the Lewis diagrams for guanine and cytosine.arrow_forwardThis molecule (is or is not) a reducing sugar. The sugar molecule is attached to the alcohol at carbon (just use a number) in the steroid structure. The lactone group is attached to carbon (just use a number) in the steroid structure. There are (just use a number) chiral carbons in this molecule. CH3 HỌ но. но, H A ÕH но ÕH CH3 • 8H2O ОН ОНarrow_forward
- How many chiral carbons does this molecule contain?arrow_forwardA chiral molecule is one that is not superposable on its mirror image, thus a chiral carbon is a carbon atom with 4 different groups attached into it. In the ff: structures below, using asterisk (*) to mark the chiral carbon. (Hint: There can be more than 1 chiral center in a molecule) он HO. он (a) (c) но OH он Lactic acid Ascorbic acid (vitamin C) он он но. (b) (d) HO Glyceraldehyde Estradiol (an estrogen)arrow_forwardHow many chiral carbons in the following molecule? 01 02 3 4 5 H OH Harrow_forward
- Classify the functional groups in the following steroid (choose all that apply).CHOOSE ALL THAT APPLY a. tertiary alcohol b. amide c. alkene d. secondary alcohol e. alkyne f. primary alcohol g. carboxylic acid h. aldehyde i. ester j. ketonearrow_forwardTo Which category does the molecule below belong? A) Protein B) alcohol C) aromatic hydrocarbons D) carbohydrate PLEASE HELP ME UNDERSTAND PLEASE PLEASE There is an image attached!arrow_forwardOrganic Chemistry HW: CANNOT BE HAND DRAWN 2,6-dimethyloct-2-ene Provide a detailed typed explanation of Stereoisomers show the expanded structure of your molecule. Calculate the maximum number of possible stereoisomers of your molecule using the following formula: Maximum number of possible stereoisomers = 2n (where n= the number of chiral carbons in your molecule). This calculation does not include E- or Z- isomers for any compounds containing double bonds Type or using a computer program "draw" the possible stereoisomers of the molecule. Note that E-, Z- isomers of each stereoisomer are also possible and would not be accounted for by the formula above; draw any E- or Z- isomers.arrow_forward
- a) Determine the absolute configuration of the chiral carbons in the following molecule: CHO Н — ОН но— H- ČH2OH b) Identify the polar and nonpolar parts of the assembly. Identify the pocket and the two bilayers that make up the assembly. Omm wO Onn w Omn Omm Omm wmO Omm wmarrow_forwardConsider the aldohexose (monosaccharide) shown below in a line structure. Which of the following correctly designates the absolute configuration (R or S) of each chiral carbon in this molecule? Но E OH OH O2R, 3S, 4R, 5R O 2R, 3R, 4S, 5R O 2R, 3R, 45, 5S O25, SR, 4S, 55 O2R, 3R, 4R, SR O2S, 3S, 4S, 5R O2S, 35, 4R, 5R O2S, 3R, 4R, 5S O2S, 3R, 45, 5S O 2R, 35, 4S, 5R O2R, 35, 4R, 5S O2R, 35, 45, 5S O2S, 3S, 4R, 55 O2S, 3R, 45, 5R O 2R, 3R, 4R, 55 O 25, 3R, 4R, 5R OH OH 0arrow_forwardChiral Carbons print/draw the expanded structure of 4-bromo-4-ethylhept-1-ene. Determine if your molecule contains any chiral carbons. If there are chiral carbons in your molecule, circle or highlight all of them. If your molecule does not contain any chiral carbons explain why none of the carbons are chiral.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning