
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
The data is for the molecular formula C8H8O3 (vanillin) with a mass of 152. Analyze the Mass spec data (include a table listing the molecular ion, base peak, and any other relevant fragments) – also include structures of fragments.
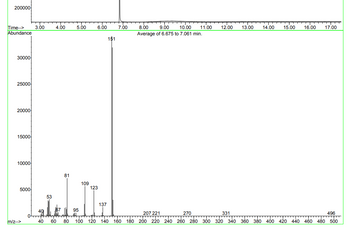
Transcribed Image Text:200000
Time-->
Abundance
30000-
25000
20000
15000
10000
5000
m/z-->
3.00 4.00
0TTT
40
53
81
5.00
109
123
6.00
137
151
7.00
8.00 9.00 10.00 11.00 12.00 13.00 14.00 15.00 16.00
Average of 6.675 to 7.061 min.
17.00
67
95
207 221
-----------
270
331
60 80 100 120 140 160 180 200 220 240 260 280 300 320 340 360 380 400 420 440 460 480 500
496
T
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Let's look at the electron-ionization Rel. Intensity 100 80 60 40 20 0.0 10 Ethanol, ¹2C₂ ¹H6 ¹60 (mass = 46) 20 a. Identify the molecular ion pick. mass spectra of ethanol (El-MS) b. Identify the base pick. Ethanol MASS SPECTRUM 30 m/z C. What type of species are detected by the detector? 40 50arrow_forwardAssign molecules B, C, D and F to the correct mass spectrum and provide the structure of thebase peak. draw the assigned moleculesarrow_forwardConsider the spectra below of an unknown compound. 100 80 21% abundance 1.2% abundance 60 40 86 20 0.0- 15 30 45 60 75 90 m/z INFRARED SPECTRUM 0.0 0.4- 02 3000 2000 1000 Wavenumber (cm 1) Relative Tranamitance Rel. Intensityarrow_forward
- which of the following statements is incorrect? A. a conventional mass spectrometer does not use a spectrophotometric detectorB. a conventional mass spectrometer does not always require high purity samplesC. a mass spectrum shows no signals due to uncharged speciesD. a conventional mass spectrometer uses high energy UV radiationarrow_forwardIn the mass spectra below, determine the Base Peak, the molecular ion peak and indicate the fragmentation process of ions marked with their respective value. 100 Mass Spectrum CH3 105 80 60 40 M 134 20 C3H100 40 80 120 160 200 240 280 m/e 100- 80 91 Mass Spectrum 60 134 40 20 149 C10 H15N 40 80 120 160 m/e 200 240 280 % of bese peak % of base peakarrow_forwardDetermine the spec unkownarrow_forward
- Please don't provide hand wriitten solution....arrow_forwardHelp figuring out how to solve to find the molecule. Please include work, thank you!arrow_forwardConsider the mass spectrum below. 100 80- 60- 20 0.0- 0.0 60 100 miz SDBS, National Institute of Advanced Industrial Science and Technology Which peak is the molecular ion? Choose one OA OB OD. OGarrow_forward
- You have analyzed Fe2+ in a dietary supplement Hemofer with VIS spectroscopy and obtained the following results, see table 1. A total of 10 tablets have analyzers. Quantify the amount of Fe2+ mg and state with 98% confidence interval. Make sure to include all steps required in the quantification calculation. Also test statistically! Evaluate your result. Did you get a good result or not?, Did something go wrong and if so what could be the reason? Use analytical and statistical concepts. Nutritional information per tablet Iron 13.5 mg Vitamin C 40 mgarrow_forward1. The mass spectrum of 3-hexanol is given below. 100 Intensity 55 80- 60- 40 40 20 20 59 73 3-Hexanol El MW = 102 OH 0 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 m/z (a) Rationalize the small size of the molecular ion peak at m/z 102. (b) Assign structures to all the labeled species and rationalize their formation.arrow_forwardI know the answer is D, but I dont know why. Can you cleave anywhere on the molecule, or only on certain areas? not certain of the rules. Is abundance the m/z (molar mass) of the molecule? I calculated 102 for the molar mass , but that is not one of the options to choose as an answer. Please explain plainlyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY