
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I need help finding the groups, chemical shift, integration, splitting, num of neighbors and fragments for C6H10O4
and draw the structure and match the peaks to it with protons
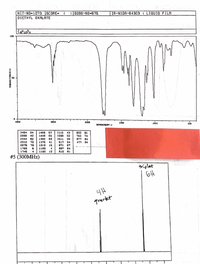
Transcribed Image Text:HIT-NO-1073 SCORE-
SDBS-NO-575
IR-NIDA-64303 : LIQUID FILM
DIETHYL OXALATE
L00
4 D00
3000
2000
1000
5DO
NAVENUNI ERI
3484
2988
802 B1
762 74
84
1459
1448
67
55
1112
1098
43
52
42
2944 02
2910 70
2879 79
1769
1746
1382
1376
1916
55
1011
26
84
67
577 84
817
871
41
477
84
16
BATT
4
DBIT
12
6
157
04
a12
TB
#5 (300MHZ)
triplet
6H
quarket

Transcribed Image Text:eport your
integration*, fragments, etc.
MR analyses nere.
abulate, chemical shin, spitting, neighbonng protons,
Group chem.Shift lepn ) Intearation Splitting tof Neigle His Fragments.
4.
Conclusion:
Draw Structure and match the peaks with the protons.
Expert Solution
arrow_forward
Step 1
The structure of the following is:
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The mass spectrum of n-octane shows a prominent molecular ion peak (m>z 114). There is also a large peak at m>z 57,but it is not the base peak. The mass spectrum of 3,4-dimethylhexane shows a smaller molecular ion, and the peak atmass 57 is the base peak. Explain these trends in abundance of the molecular ions and the ions at mass 57, and predict theintensities of the peaks at masses 57 and 114 in the spectrum of 2,2,3,3-tetramethylbutane.1arrow_forwardHow do I predict the M, M+2. and M+4 with this information?arrow_forward6.arrow_forward
- Mass Spectrum 1000 - 800.0 600.0 400.0 200.0 0.000- 50.00 100.0 m/z 150.0 200.0 lamalan saldassallaalan C-H;BRO, D00 3000 2000 1000 500 HAVENUNBERIl Abundancearrow_forwardArrange in ascending order of chemical shift (δ) the protons indicated in thestructure below. Fill in the empty boxes with the corresponding letters. b. Explain your choice of the relative order of the chemical shifts of the protons Hc andHD.arrow_forward. Deduce the structure of an unknown compound with the molecular formula of C5H₁2O using the information given by its infrared spectrum. ANSWER A) НО. B) D) Intensity Frequency (peak): (cm): 3300 m EEEEE m m m m m 2900 2800 1465 1450 1375arrow_forward
- I need help with thisarrow_forwardThe following molecular ion isotopic clusters correspond to derivatives of benzene (C6H6) for which one or more of the hydrogens have been replaced with chlorines or bromines. What molecular formula corresponds to each mass spectrum? . 100- 80- 60- 40- Relative intensity 20- -M+6 -M+5 -M+4 M+3 M+1 M+2 M+0 (a) -M+6 -M+5 -M+4 M+3 M+2 M+1 -M+0 (b) M+6 M+5 M+4 M+3 -M+2 -M+1 -M+0 (c) M+6 -M+5 M+4 M+3 M+2 -M+1 -M+0 (d) M+6 M+5 -M+4 -M+3 -M+2 M+1 -M+0 (e)arrow_forward1. The mass spectrum of 3-hexanol is given below. 100 Intensity 55 80- 60- 40 40 20 20 59 73 3-Hexanol El MW = 102 OH 0 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 m/z (a) Rationalize the small size of the molecular ion peak at m/z 102. (b) Assign structures to all the labeled species and rationalize their formation.arrow_forward
- Using the following NMR spectrum please identify what molecules are present along with their chemical shifts and their multiplicity.arrow_forward(Figure 1) Figure (a) (c) (CH3)3C H (b) (d) 1 of 1 Part A Predict the number of 13C NMR signals in the proton-decoupled spectrum of (a). Express your answer numerically using one significant figure. 15| ΑΣΦ Part B ? Predict the number of 13C NMR signals in the proton-decoupled spectrum of (b). Express your answer numerically using one significant figure. ΠΫΠΙ ΑΣΦ ?arrow_forwardDetermine the molecular ion peak in the mass spectra below.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY