
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Fully annotate ms. Using specific pieces of data from the spectra please discuss how you determined the identity of the terpene unknown. Your answer should be 1-2 paragraphs.
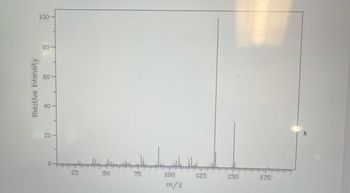
Transcribed Image Text:Relative Intensity
100-
80
60
0
20-
0
25
50
75
100
m/z
125
150
175
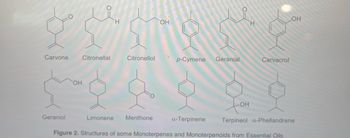
Transcribed Image Text:bil
Carvone
Geraniol
OH
Citronellal
H
Citronellol
Limonene Menthone
OH
p-Cymene
you
Geranial
OH
H
Carvacrol
LOH
a-Terpinene Terpineol a-Phellandrene
Figure 2. Structures of some Monoterpenes and Monoterpenoids from Essential Oils
Expert Solution
arrow_forward
Step 1: Description of the question
A question based on mass spectroscopy. For the given mass spectrum, the most appropriate molecule from the given options is identified.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. List the integration of all peaks as a ratio. For example, 4-hydroxy-4-methyl-2-pentanone would have a ratio of 1:2:3:6.How does the integration of the peaks correlate to the number of hydrogens present in the molecule? 2. For each peak, draw a partial structure that uses all three pieces of information (chemical shift, integration, splitting patterns). Make sure that you highlight the hydrogen atom or atoms that are responsible for the signal. An example of this is: -CO-CH2-CR2- 3. Based on the chemical shifts, what functional groups are present in your compound? For each, correlate the functional group with the chemical shift of the identifying peak.arrow_forward. Calculation of HDI for each compound show work . Label and assign all the functional groups in the diagnostic region of the IR . Label and assign all protons in the NMR on your drawing ( clearly annotate each set of chemically non equivalent protons on your proposed structure and assign their signal on the 1H-NMR spectrum. .what lead to the proposed structure using the HDI,IR AND 1-H-NMR data provided.arrow_forwardINSMART Board El Predict the H NMR spectrum of product. groups and label H₁, H³, H..... of each signal 2. Estimate chemical shift range 3. Predict splitting pattern of each signal 1. Identify unique Harrow_forward
- Model 3: Ionization and Fragmentation Most mass spectrometers accelerate a molecule by first turning it into an ion, then using an electric field to accelerate it toward the detector. During standard ionization, a molecule loses one electron, the electron that is easiest to remove. The following is a hierarchy of electrons from easiest to hardest to remove: Electron in a lone pair (easiest) Electron that is part of a double bond (pi bond) Electron in a single bond (hardest) Knocking off an electron to make a +1 ion can be a harsh process. This harsh treatment often results in a broken bond, generating two smaller pieces, a +1 ion and a neutral fragment. (Note that only the ion is accelerated and detected.) ● 7. ● Critical Thinking Questions 6. If acetone is ionized, which electron is most likely to be knocked off? Replace this electron with a + (to indicate the missing electron) on the structure of acetone → The ion you drew above is called the "molecular ion" of acetone. It is…arrow_forwardvalues : Experimental values of IR Peaks compared to literature values for Benzoic Acid (ATR method) Bond Stretch Functional group Experimental Peak(cm-1) Literature Peak(cm-1) O-H Carboxylic acid 2800 3200-2200 C=O Carboxylic Acid 1674.9 1677.5 C=C (ring) Aromatic stretch 1579.5 1581-1418.5 C-H Aromatic Sp2 3100 C-O stretch 1285.5 O-H Alcohol 929.89 table 3: Experimental values of IR peaks compared to literature values for 2-Naphthol Bond Stretch Functional group Experimental Peak(cm-1) Literature Peak(cm-1) O-H Alcohol 3219.1 3400-3080 C-C Aromatic (ring) stretch 1507.6 C=C Aromatic stretch 1597.7 1627.3-1377.8 C-H Aromatic Sp2 3050 C-O Secondary alcohol stretch 1168.9 IR results used to prove that the compounds are effectively separated. Discuss on bothdiagnostic peaks and fingerprint region.arrow_forwardImagine that you were given an unidentified aldehyde and performed another Wittig reaction in lab. Use the given data to answer the questions below and identify your original aldehyde. 8 5 R A H 7 + 15 1 C/O (C6H5)3P- Below is shown the ¹H spectrum for the pure alkene product of this experiment. Interpret the signals to identify "R" by assigning each hydrogen by chemical shift, multiplicity (splitting), integration, and any other significant features. 6 A B -5 NaOH 4 PPM (b) One possible alkene product is shown in the reaction above. Draw the other alkene product R 3 H 2 + (C6H5)3PO 2 1 6 1 (a) Label which proton in the product above corresponds to each signal A and B. Explain your assignment and what this tells you about the "R" group. (c) What was your aldehyde starting material? (What is "R"?) 1arrow_forward
- From the spectra A-J and in the NMR Spectra tile, select the letter that corresponds to 1. methyl butanoate2. benzaldehyde3. 1-chlorobutane4. 1-chloro-2-methylpropane5. butan-2-one6. propan-2-ol7. propanalarrow_forwardBased on the spectra provided, draw the structure. Label each unique carbon and hydrogen with the letters A, B, C… for use in assigning NMR peaks. fill in the data table assigning peaks in each spectrum. You should assign: • All 1H NMR peaks • Significant IR peaks above 1600 cm Example of what the table should include (imagine the structure of ethanol is drawn with the CH3 hydrogens labeled as A, the CH2 hydrogens labeled as B, and the OH hydrogen labeled as C) : hydrogen proton chemical shift integration splitting pattern couples to.. A 1.2 3 triplet B B 3.7 2 quartet A C 2.6 1 singlet -arrow_forwardLabel all relevant peaks that are above the fingerprint region (>1500 cm-1) in all the IR spectra given below with the functional group that those peaks are representative of (make sure not to forget the aldehyde C–H bonds, which many IR charts do not list). Based on the table you completed with predicted Diagnostic IR peaks for each compound, and based on the IR spectra you had labeled, match each compound with its spectrum.arrow_forward
- Please circle the answersarrow_forwardConstruct a simulated 'H NMR spectrum for the given structural formula. Drag the appropriate splitting patterns to the approximate chemical shift positions; place the integration values above their associated signal. Splitting patterns and integrations may be used more than once, or not at all, as needed. Likewise, some bins will remain blank. Note that peak heights are arbitrary and do not indicate proton integrations. A HO 7 6 3 2 1 0 ppm 2H 3H 1H 5 4 Answer Bank 4Harrow_forwardurgent :i am supposed to match the peaks on the compound to where it is on the nmr spectrscopy. i started matching but am not sure if i am right, please help finish and correct if wrongarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY