
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
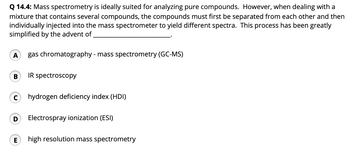
Transcribed Image Text:Q 14.4: Mass spectrometry is ideally suited for analyzing pure compounds. However, when dealing with a
mixture that contains several compounds, the compounds must first be separated from each other and then
individually injected into the mass spectrometer to yield different spectra. This process has been greatly
simplified by the advent of
A gas chromatography - mass spectrometry (GC-MS)
B
C
IR spectroscopy
hydrogen deficiency index (HDI)
D Electrospray ionization (ESI)
E high resolution mass spectrometry
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You have analyzed Fe2+ in a dietary supplement Hemofer with VIS spectroscopy and obtained the following results, see table 1. A total of 10 tablets have analyzers. Quantify the amount of Fe2+ mg and state with 98% confidence interval. Make sure to include all steps required in the quantification calculation. Also test statistically! Evaluate your result. Did you get a good result or not?, Did something go wrong and if so what could be the reason? Use analytical and statistical concepts. Nutritional information per tablet Iron 13.5 mg Vitamin C 40 mgarrow_forwardPlease do it correctly neat and cleanarrow_forwardUsing mass spectrum and H+NMR, propose a compound containg C doubled bonded to O and C bonded to H (sp3)arrow_forward
- 1. The mass spectrum of 3-hexanol is given below. 100 Intensity 55 80- 60- 40 40 20 20 59 73 3-Hexanol El MW = 102 OH 0 0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 m/z (a) Rationalize the small size of the molecular ion peak at m/z 102. (b) Assign structures to all the labeled species and rationalize their formation.arrow_forwardDetermine the molecular ion peak in the mass spectra below.arrow_forward7. Match the type of analysis output to the units appearing on its x-axis. 1H NMR spectrum IR spectrum gas chromatogram mass spectrumarrow_forward
- 27. The rule you generated in Question 14b for finding the [M] peak on the mass spectrum of an unknown molecule does not work very well if there is a Br or Cl in the molecule. Shown below are spectra of various compounds containing one Br or Cl. a. Find the [M] and [M+2]* peak on each spectrum. Relative Intensity 100- Relative Intensity 80- 60 20- 0-mmm 100- 80 60- 40 18--8163 20- 25 HS-NU-2127 0-forttitor 10 20 b. 30 50 40 50 Br pretztenyeregtelefongum 75 m/z 60 m/z Bri 70 100 (2) one Br atom. 125 effleyttim 90 80 ||||||| 100 110 Relative Intensity 100 Relative Intensity 80 60- 40 20 0-m 100- 80 60 40 20 15-MM-0968 0- spatepragning 25 115--1439 25 50 50 75 NH₂ 100 m/z mn||| 75 m/z 125 100 150 125 Write a rule for quickly identifying from a mass spectrum if an unknown molecule has... (1) one Cl atom. Note: The mass spectrum of a molecule with more than one Cl or Br can be quite complex, so we will limit cases to one Cl or Br. Write a new rule for finding the [M]* peak that takes into…arrow_forward3. Dibromocholestanol was analyzed by mass spectrometry using the electron impact (El) ionization technique. The following peaks were observed with relative intensities of ~1:2:1 Low Resolution MS (EI) m/z= 544, 546, and 548 Identify the peaks in the molecular ion pattern and which combination of isotopes produces these peaks: [M]: m/z = formula: [M+2]+: m/z = [M+4]+ : m/z = formula: formula:arrow_forward15arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY