
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
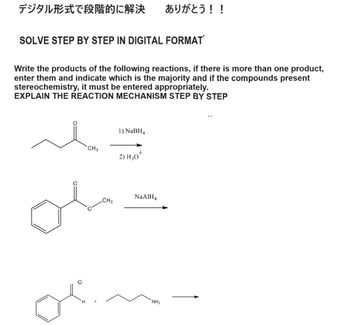
Transcribed Image Text:デジタル形式で段階的に解決 ありがとう!!
SOLVE STEP BY STEP IN DIGITAL FORMAT
Write the products of the following reactions, if there is more than one product,
enter them and indicate which is the majority and if the compounds present
stereochemistry, it must be entered appropriately.
EXPLAIN THE REACTION MECHANISM STEP BY STEP
مله
1) NaBH4
CH3
2) H₂O+
CH3
NaAlH4
NH₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- Propose a mechanism for the reaction of 3-methyl-1-butene with HBr in peroxide. Name and draw the product and determine the stereochemistry. Use arrows to show movement of electrons and use words to describe the elementary steps.arrow_forwardPredict the product(s) and provide the name(s) of the products for the following reactions: H X $R= Br Br I KOH ethanol heat KOH EtOH Heat CH3CH₂O- CH3CH₂OHarrow_forwardデジタル形式で段階的に解決 ありがとう!! SOLVE STEP BY STEP IN DIGITAL FORMAT Write the products of the following reactions, if there is more than one product, enter them and indicate which is the majority and if the compounds present stereochemistry, it must be entered appropriately. EXPLAIN THE REACTION MECHANISM STEP BY STEP HCI + HS. SH + H₂ Ni Raneyarrow_forward
- 人工知能を使用せず、 すべてを段階的にデジタル形式で解決してください。 ありがとう SOLVE STEP BY STEP IN DIGITAL FORMAT DON'T USE CHATGPT 3.- Provide the products of each reaction and say what type of product it corresponds to (SN1 or SN2). As well as the reaction mechanism from which they are formed. ゾ Br Na CN CH3CH2OH Na OCH₂CH3 EtOHarrow_forward6A1: Distinguish between synthetic and retrosynthetic transformations to solve problems and predict starting materials and products. Draw any retrosynthetic sequence where a hydroxyl cycloalkane retron can be retrosynthesized into an appropriate cycloalkene, where the cycloalkene can be retrosynthesized into an appropriate cycloalkane halide. While you are allowed to work out this problem synthetically, you MUST report your final response in the retrosynthetic direction using the symbology we learned in lecture/arrow_forwardPredict the major organic products of the following reaction. You do not need to include stereochemistry in your answer. If no significant reaction will occur under the conditions shown, check the box under the drawing area instead. Note: for the purpose of this problem, simple acid-base reactions between the reagents should not be considered a significant reaction. H₂N 7 + 1. LIAI H (OtBu) 2.H₂0 Click and drag to start drawing a structure.arrow_forward
- Suppose you are told that each reaction is a substitution reaction but are not told the mechanism. Describe how you could conclude from the structure of the haloalkane, the nucleophile, and the solvent that each reaction is an SN2 reaction.arrow_forward(a) In an acid-catalyzed hydration, one of the following 10 carbon alkynes is expected to produce a single hydration product? Select the correct alkyne and draw the structure of the hydration product that is formed from this alkyne. (I) 2-decyne; (II) 3-decyne; (III) 4-decyne; (IV) 5-decyne; (V) none of these will give a single hydration product. (b) The reaction shown below gives Compound X as the major product. The mass spectrum of X is shown. Br2, H20 Compound X 100 - MS-IW-5644 80 60 40 - 20 - 20 40 60 80 100 120 140 160 180 200 220 m/z Considering the reactions of alkynes and the MS data, de duce which of following structures corresponds to X: Br Br HO, IV V I II II Support your answer with a reaction mechanism for fomation of X and identification of relevant peaks in the mass spectrum. 12 Relative Intensityarrow_forwardProvide a short answer for why the secondary alkyl halide is the major product versus the tertiary alkyl halide from free radical halogention H₂C NBS H₂C, Br H₂C むーびず H peroxides Br + enantiomer + enantiomer Major Minorarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY