
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Schemes of the reaction mechanism and its result are needed.
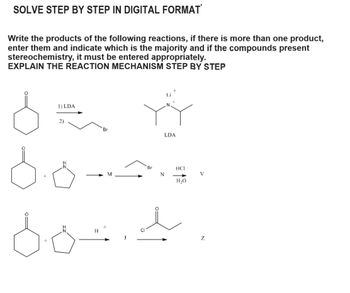
Transcribed Image Text:SOLVE STEP BY STEP IN DIGITAL FORMAT
Write the products of the following reactions, if there is more than one product,
enter them and indicate which is the majority and if the compounds present
stereochemistry, it must be entered appropriately.
EXPLAIN THE REACTION MECHANISM STEP BY STEP
1) LDA
2)
H
Br
LDA
Br
HCI
N
V
H₂O
Z.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- What are the units for each parameter? maximum velocity Michaelis-Menton constant catalytic efficiency catalytic constant 111 M/S M 1/(Ms) 1/s M/s 100/(Ms)arrow_forwardIn a certain collision, an energy equivalent to 400 kJ mol−1 is delivered; the energy needed to break a bond is 350 kJ mol−1; there are 5 relevant molecular modes. What is the value of the P steric-factor for the reactive encounter? (Answer: p = 1.3 x 10-4)arrow_forwardA manometer was connected to a bulb containing nitrogen under slight pressure. The gas was allowed to escape through a small pinhole, and the time for the manometer reading to drop from 65.1 cm to 42.1 cm was 18.5 s. When the experiment was repeated using a fluorocarbon gas, the same fall took place in 82.3 s. Calculate the molar mass of the fluorocarbon.arrow_forward
- Ll.52.arrow_forward2) Consider the following reaction and associated rate law: X (g) + Y(g) → Z Rate = k[X] [Y] b Use the data in Table 1 in the following problems. Table 1 Reaction Data [X]。 (M) [Y]。 (M) Rate (M/s) 0.882 0.217 283. 07 0.882 0.451 624. 47 0.882 0.572 820.57 0.882 0.882 1308. 22 0.228 0.882 124.96 0.442 0.882 382. 56 0.67 0.882 804. 48arrow_forwardSuppose the concentration of a solute decays linearly along the length of a container according to c(x) = c0 - αc0x, where c0 is the concentration at x = 0. Calculate the thermodynamic force on the solute at 25 °C and at x = 10 cm and 20 cm given that the concentration falls to 1/2c0 when x = 10 cm. Hint: Start by finding the value of α.arrow_forward
- The following sequence of steps has been proposed for the overall reaction between H2 and Br2 to form HBr:(1) Br2(g) 2Br(g)Write the overall balanced equation and show that the overall Qc is the product of the Qc’s for the individualsteps.arrow_forward4. Which of the following best describes the timescales of fluorescence and phosphorescence? (b) Tfluorphos (d) there is no general trend Tfluor > Tphos Tfluor Tphos In 1-2 sentences, explain your reasoning:arrow_forward6. Suppose that a gas phase reaction: 2A(g) 2B(g) +C(g) follows second order kinetics and goes to completion. If the reaction is allowed to proceed in a constant volume vessel at an initial pressure of 4 bar (only A is initially present). The total pressure of the reaction mixture at t= o is: * 4 bar 9 bar O 3 bar 6 bararrow_forward
- According to the nature or physical mechanism of separation, the separation process can be categorized into three main types. Write down in details what are the three main types.arrow_forward7) For the reaction CO(g) + 3 H2(g) = H2O(g) + CH4(g), Kc = 190 at 1000 K. If a vessel is filled with these gases such that the initial concentrations are [CO] = 0.036 M, [H2] = 0.045, [H2O] = 0.020, 7) %3D %3D %3D and [CH4] = 0.031, in which direction will a reaction occur and why? A) it is at equilibrium because Q = 190 B) toward products because Q = 4.1 C) toward reactants because Q = 0.24 D) toward products because Q = 0.38 %3D %3D %3D %3D E) toward reactants because Q = 61 %3Darrow_forward6. Five reaction mixtures containing equal concentrations of an enzyme are made up to the substrate concentrations [S] indicated in the table below and the initial rates of the reaction (V) are measured. The experiment is repeated with an enzyme inhibitor I present at 0.22 mM in each reaction mixture. (a) Determine Km for the substrate and K, for the inhibitor. (b) Can you say anything about the type of the inhibitor? [S], mM V (µmol/min) (no I) V (µmol/min) (+ 0.22 mM I) 0.1 28 17 0.15 36 23 0.2 43 29 0.5 65 50 0.75 74 61arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY