
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
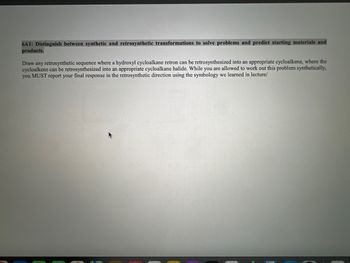
Transcribed Image Text:6A1: Distinguish between synthetic and retrosynthetic transformations to solve problems and predict starting materials and
products.
Draw any retrosynthetic sequence where a hydroxyl cycloalkane retron can be retrosynthesized into an appropriate cycloalkene, where the
cycloalkene can be retrosynthesized into an appropriate cycloalkane halide. While you are allowed to work out this problem synthetically,
you MUST report your final response in the retrosynthetic direction using the symbology we learned in lecture/
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4. The following transformations require multiple steps. Show how to accomplish each transformation. You may use any necessary reagent containing 4 carbon atoms or fewer. Š Br NH₂ N + Brarrow_forwardOH OH The transformation above can be performed with some combination of the reagents listed below. Give the necessary reagents in the correct order for each transformation, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A t-BuOK D H2, Pt G B C HBr, ROOR conc. H2SO4 E F H2, Lindlar's cat. HBr H 1) BH3.THF; 2) H2O2, NaOH HC=CNa TsCl, pyridinearrow_forwardIdentify reagents that can be used to achieve the following transformation: H₂O, H₂SO4, HgSO4 The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A D 1) 03 2) DMS G سعد 1) xs NaNH2 2) H₂O B Br2 E H H₂, Lindlar's catalyst H t-BuOK с HC=CNa F 1) R₂BH 2) H₂O2, NaOH I H₂, Pdarrow_forward
- Draw a step-by-step mechanism for the transformation shown below (no additional reagents are needed), showing all electron flow with arrows.arrow_forwardor the following carbocation determine if it is likely to rearrange, and if so, select the correct carbocation rearrangement: о ЊС Н.С O CH₂ CH₂ O There is no rearrangement for this carbocation. О HỌC CH НС H₂C сх-сно H HỌC CH X _CH2 HỌC HỌC y J Н CH₂ зорил CH₂ НС- - H.C H.C CH 3 -C+ Н.С ЊС CH₂ CH₂ CH CH₂ CH CH₂ CH₂arrow_forwardThe following reaction is similar to the dehydration of 2-methylcyclohexanol. However, a significant amount of a tetrasubstituted alkene is expected. Predict the structure for this tetrasubstituted alkene. Also, using curved arrows, provide a reasonable mechanism for its formation. Но. H3PO4 ??? A (-H2O)arrow_forward
- Which of the following reagents is best to achieve the given transformation? H₂C 0=s=0 0000 → KOtBu OCH3OH NaOH TsCI, Pyarrow_forwardPredict the major products of this reaction. ХХ Cl₂ hv ? Draw only the major product or products in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If there will be no products because there will be no significant reaction, just check the box under the drawing area and leave it blank. Note for advanced students: you can ignore any products of repeated addition. Click and drag to start drawing a structure. E 'n X 0:0 toarrow_forwardWhen the following alkene A was treated with sulfuric acid in water, the cyclohexanol product B was formed. Using curved arrows give the mechanism for this reaction, including any regioselectivity or stereoselectivity. CH3 CH3 OH H2SO4 CH3 A H₂O CH3 ៣ Barrow_forward
- Draw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. CH3 CH3 H3PO4 HO, + H3C CH3 HO HO First stage in synthesis of the epoxy and polycarbonate ingredient bisphenol-A You do not have to consider stereochemistry. Include all valence lone pairs in your answer. In cases where there is more than one answer, just draw one.arrow_forwardComplete the following reactions by providing the missing product(s). Determine what mechanism operates in each case (SN1, SN2, E1, or E2). Show the stereochemistry of the product(s) where appropriate. If more than one product is formed circle the major one.arrow_forwardFill in the major products to complete the transformations. Be sure to pay carefulattention to stereochemistry where appropriate. If the major product is a pair of enantiomers or diastereomers be sure to draw them both. And indicate whether you have drawn an enantiomer or a diastereomer. (Don't forget about rearrangement).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY