
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Review I Constants I Periodic Tabl
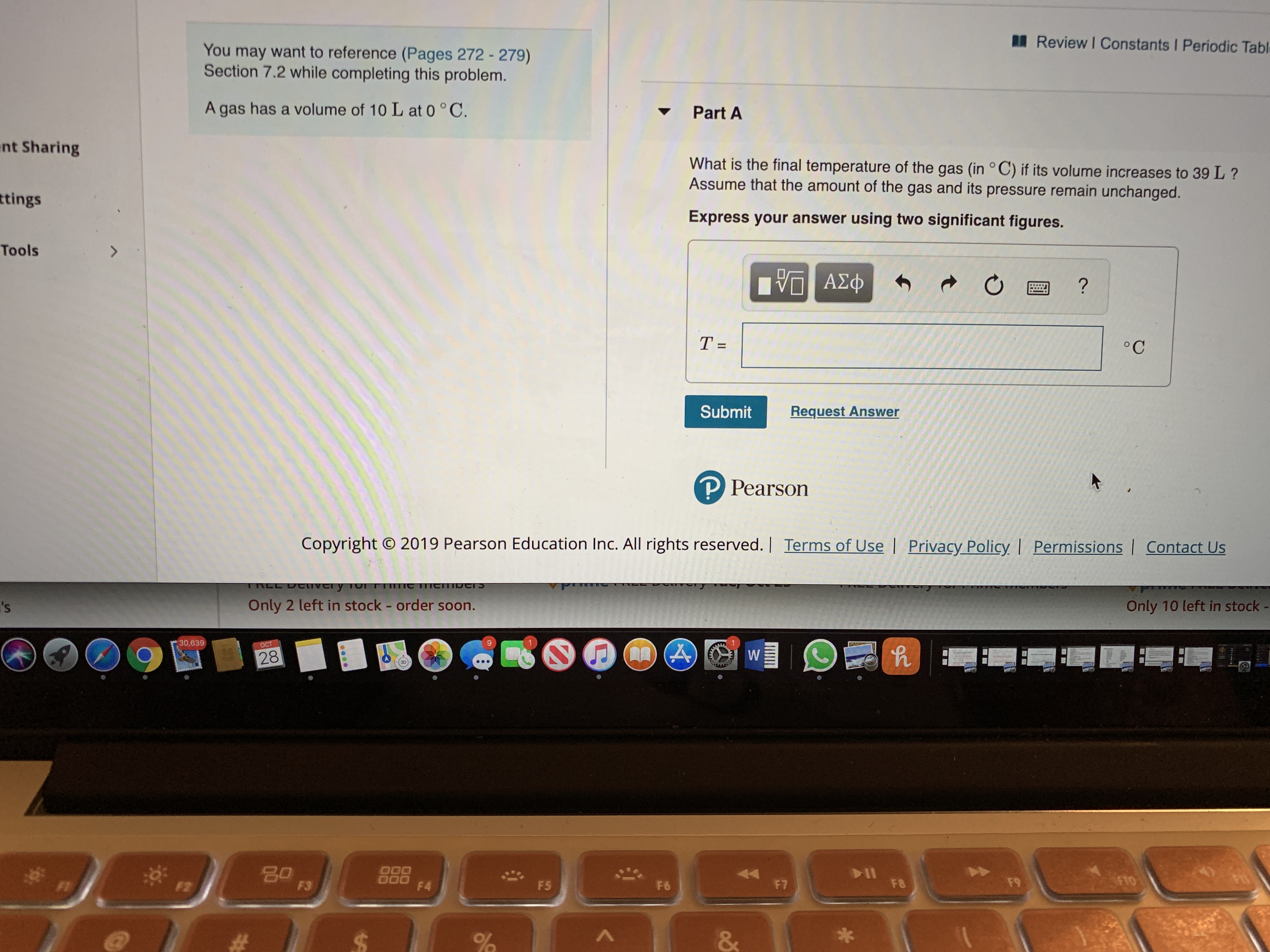
You may want to reference (Pages 272- 279)
Section 7.2 while completing this problem.
Part A
A gas has a volume of 10 L at 0 °C.
ent Sharing
What is the final temperature of the gas (in °C) if its volume increases to 39L?
Assume that the amount of the gas and its pressure remain unchanged.
tings
Express your answer using two significant figures.
Tools
7
VA
°C
T =
Request Answer
Submit
P Pearson
Permissions Contact Us
Copyright O 2019 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy
TITTC TTTCmocS
TNEE DEver y To
Only 10 left in stock -
Only 2 left in stock - order soon.
's
1
A
W
30,639
ост
2P
28
FTO
go
O00
O00
F4
F9
F8
F7
F6
F5
F3
F2
&
%
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- A mixture of argon and methane gases, in a 8.78 L flask at 73 °C, contains 7.99 grams of argon and 1.48 grams of methane. The partial pressure of methane in the flask is atm and the total pressure in the flask is __________atm.arrow_forwardA sample of a gas mixture contains the following quantities of three gases. compound mass CO 1.08 g CO2 1.84 g SF6 1.54 g The sample has: volume = 2.50 L temperature = 16.6 °C What is the partial pressure for each gas, in mmHg? What is the total pressure in the flask? CO |mmHg CO2 mmHg SF6 mmHg total |mmHg Show Approach Hide Tutor Steps Submitarrow_forwardA basketball is inflated to 9.0 psi above atmospheric pressure of 14.7 psi (a total pressure of 23.7 psi). The diameter of the basketball is 10 inches exact. a. What is the pressure of the air in the basketball, expressed in atmospheres? b. What is the volume (in liters) of the basketball? The volume of a sphere= 4/3 pie r^3arrow_forward
- [References] Use the References to access important values if needed for this question. A sample of an unknown gas is found to have a density of 1.97 g/L at a pressure of 0.762 atm and a temperature of 51 °C. Assume ideal behavior. The molar mass of the unknown gas is g/mol. Submit Answer Try Another Version 1 item attempt remaining Visited Previous Next 10 10 MAR étv W 280arrow_forwardPart C A sample of neon initially has a volume of 2.50 L at 20. °C. What final temperature, in degrees Celsius, is needed to change the volume of the gas to each of the following, if pressure and amount of gas do not change? 8.50 L Express your answer with the appropriate units. HA ? T- Value Units Submit Request Answer Part D 3750 mL Express your answer with the appropriate units. HẢ ? T- Value Units Submit Request Answerarrow_forwardPlease send me the question in 30 minutes it's very urgent plzarrow_forward
- A deep-sea diver uses a gas cylinder with a volume of 10.0 L and a content of 50.5 g of O2 and 32.0 g of He. Part A Calculate the partial pressure of each gas and the total pressure if the temperature of the gas is 15 °C. Express the pressures in atmospheres to three significant digits separated by commas. ΠΙ ΑΣΦ Po₂, PHe, Ptotal : = Submit Provide Feedback Request Answer ? atm Next >arrow_forward12.arrow_forwardEsc Russell 2000 -1.51% 1 A A balloon is filled to a volume of 1.50 L with 2.50 moles of gas at 25 °C. With pressure and temperature held constant, what will be the volume of the balloon if 0.20 moles of gas are added? 1 @ 2 W S # 3 G E D $ 4 R F4 LL F H DII % 5 Q Search T G ^ Question 16 of 30 6 a Y & 7 H PrtScne 8 Home J 9 Encharrow_forward
- A gas mixture with a total pressure of 2300 torr is used by a scuba diver. If the mixture contains 2.5 moles of helium and 5.0 moles of oxygen, what is the partial pressure, in torr, of each gas in the sample? Express your answer in torr to two significant figures. PHe = _____ PO2 _____arrow_forwardIn the open ended manometer (open to the atmosphere) pictured below, what is the pressure of the gas in the bulb if the pressure of the atmosphere is 755 mm Hg and h = 55 mm Hg? Input your answer in mm Hg.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY