
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please help! I need answers I keep getting the wrong answers.
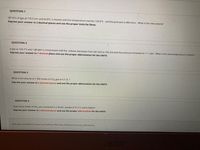
Transcribed Image Text:QUESTION 1
201.61L of gas at 770.5 torr and 63.8°C is heated until the temperature reaches 134.8°C. and the pressure is 889.2torr. What is the new volume?
Express your answer to 2 decimal places and use the proper Units for litres.
QUESTION 2
A gas at 104.7°C and 1.88 atm is compressed until the volume decreases from 581.6ml to 105.2ml and the pressure increases to 11.1 atm. What is the new temperature in Kelvin?
Express your answer to 1 decimal place and use the proper abbreviation for the UNITS
QUESTION 3
What is the volume of 1.595 moles of CO2 gas at S.T. P.?
Express your answer to 2 decimal places and use the proper abbreviation for the UNITS
QUESTION 4
How many moles of NH3 are contained in a 30.83L sample at 91.5°C and 6.28atm?
Express your answer to 2 decimal places and use the proper abbreviation for the UNITS
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
ace
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I'm resending this question because the image on the last one was blurry. I wrote the prompt and steps and helpful conversions to figuring out the real world problem on my scratch paper, how do I solve for this problem?arrow_forwardNo work needed just answers label the answers with questions so I know directions are all the samearrow_forward10 11 12 13 14 15 16 17 18 19 The particles in a gas vibrate faster than the particles in a liquid. O True O False 5.arrow_forward
- True or false? (No need explanation) different types of gloves protect against different types of chemicals. The most likely route of entry for exposure to chemicals is through your nose and mouth. Never expose yourself to any meterial unless you know exactly what it is, how it can hurt you and what you need to protect yourselfarrow_forwardSuppose that you heated the hydrated copper (II) sulphate in a test tube, instead of a beaker. How might this affect your results? 4 MAY 19 ... MacBook Pro & * %23 $ 2 3 4 5 6 7 E Y U G H J K C V M MOSISO command option command レーarrow_forwardGases You should be able to answer this prompt using 10 sentences or less. Gases are very important for life in terms of respiration and photosynthesis. However, gases play other critical roles in our daily lives. Think about the past month. Did you use any product that is a gas at room temperature? What was the product or substance? State one chemical or physical property of the substance that is unique because it is a gas.arrow_forward
- A chemistry student weighs out 0.117 g of hypobromous acid (HBrO) into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1600 M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the equivalence point. Be sure your answer has the correct number of significant digits. mL 0 x10 Xarrow_forward8 - 14 pleasearrow_forwardQuestion 12arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY