
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
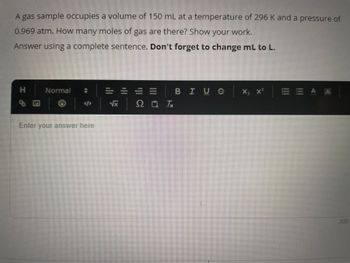
Transcribed Image Text:A gas sample occupies a volume of 150 mL at a temperature of 296 K and a pressure of
0.969 atm. How many moles of gas are there? Show your work.
Answer using a complete sentence. Don't forget to change mL to L.
H
0
Normal
@
Enter your answer here
H
Qaz
BI U 6
X, X²¹
EEAA
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A sample of nitrogen gas at 310 K and 1.01 atm occupies a volume of 1.51 L. If the gas is compressed into a smaller volume, while at the same time it is cooled to a lower temperature, the final gas pressure ? will be lower than 1.01 atm. ? will be higher than 1.01 atm. ? could be higher or lower than 1.01 atm depending on the final volume and temperature.arrow_forwardA sample of gas as 230 K changes temperature until it reaches 59°C. The initial volume is 3.5 L, what is the final volume in liters? Show work on your paper. In the box below, enter your numerical answer only, in L.arrow_forwardA 532 mL sample of neon gas has a pressure of 4.7 atm which is then reduced to 5.4 atm. What is the new volume of the neon gas? Show your work and box your final answer.arrow_forward
- 1. At 27°C and 720 Torr, a certain gas sample has a volume of 765 mL. a. What would be its new volume if the temperature and pressure were changed to 57°C and 792 Torr? Hint: Use Combined Gas Law. b. How many moles are present in that gas sample? Hint: Use Universal Gas Law.arrow_forwardHow many gas molecules are present in 727 mL of propane (C3H8) at 27 °C and 494 torr pressure? Enter using scientific notation. How many atoms are present? Enter using scientific notation. What would the volume of the sample be at STP? Enter using decimal notation.arrow_forwardSulfur hexafluoride gas is collected at 3.0 °C in an evacuated flask with a measured volume of 30.0 L. When all the gas has been collected, the pressure in the flask is measured to be 0.270 atm . Calculate the mass and number of moles of sulfur hexafluoride gas that were collected. Round your answer to 3 significant digits. mass: mole: molarrow_forward
- 2. During the experiment, you collect the following data: Mass of flask: 80.001 Mass of flask + unknown condensed liquid: 80.452 Temperature of water bath: 100 °C Pressure of gas in our system: 0.998 atm Volume of gas in our system: 265 mL How many moles of gas were produced? Hint: Do not forget to convert units as needed. Group of answer choices: A) 0.00864 moles B) 32.2 moles C) 0.0322 moles D) 8.64 molesarrow_forwardA balloon contains 0.154 mol of gas and has a volume of 2.61 L . If an additional 0.200 mol of gas is added to the balloon (at the same temperature and pressure), what will its final volume be? Express your answer in liters to three significant figures.arrow_forwardA gas sample initially at 1 atm increases in pressure to 4.51 atm as the volume shrinks to 5.6 L. What was the initial volume? Show work on your paper. In the box below, enter your numeric answer only.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY