
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
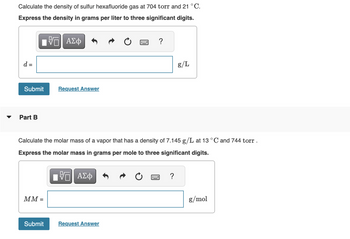
Transcribed Image Text:Calculate the density of sulfur hexafluoride gas at 704 torr and 21 °C.
Express the density in grams per liter to three significant digits.
d =
17 ΑΣΦ
VO
Submit
Part B
MM =
Request Answer
Submit
Calculate the molar mass of a vapor that has a density of 7.145 g/L at 13 °C and 744 torr.
Express the molar mass in grams per mole to three significant digits.
ΠΫΠΙ ΑΣΦ
?
Request Answer
g/L
?
g/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A sample of neon gas occupies a volume of 9.78 L at 57.0°C and 0.940 atm. If it is desired to increase the volume of the gas sample to 11.9 L, while increasing its pressure to 1.31 atm, the temperature of the gas sample at the new volume and pressure must be _________°C.arrow_forwardWhat volume of O₂ gas, measured at 782 mmHg and 26 °C, is required to completely react with 54.6 g of Al? Express the volume in liters to three significant figures. 17 ΑΣΦ V= Submit Request Answer Larrow_forwardA colleague of yours takes a bunch of pressure readings while you are gone. You return to realize that he has invented a new unit: his body mass of 180lb per 1mile?. He calls this unit Bob per Bob's Block. 1mile = 63360 inches. What is the pressure of 9 Bobs per Bob's block in a real unit of pressure, pounds per square inch (PSI)? Use just one sig fig in your answer: x10^ PSIarrow_forward
- Assume that an exhaled breath of air consists of 74.6 % No. 15.5 % O2, 3.6 % CO2, and 6.3 % water vapor. Correct Part E Previous Answers If the volume of the exhaled gas is 460 mL, and its temperature is 37 °C calculate the number of moles of CO, exhaled.. Express your answer using two significant figures. Ο ΑΣΦ CO Submit Previous Answers Request Answer molarrow_forward10001 me r 9 е 9.38 Constants Part A Assuming all gases are at the same temperature and pressure, how many milliliters of hydrogen iodide are produced from 165 mL of H2 ? H2 (g) +I2(g)→2HI(g) Express your answer with the appropriate units. HÁ VHI Value %3D Submit Previous Answers Reguest Answer X Incorrect; Try Again Provide Feedbackarrow_forwardCanvas Hyperbaric oxygen chambers providing pure oxygen (O2) at pressures greater than 1 atm are used to treat divers suffering from decompression sickness as well as patients with thermal burns and carbon monoxide poisoning. What is the pressure in a chamber with a volume of 2,500 L containing 5,000 g of oxygen at 298 K? Edit Format Table BIU A v v T?v * 12pt v Paragraph v O words The atmospheric pressure in the eye of a hurricane is found to be 26.6 in of mercury. The pressure in the eye of this hurricane is MacBook Pro %23 2 3 4. 5 7 8arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY