
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
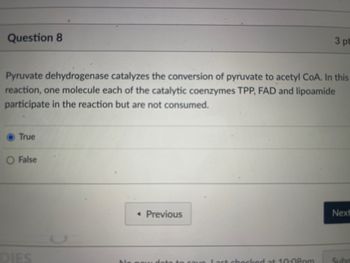
Transcribed Image Text:Question 8
Pyruvate dehydrogenase catalyzes the conversion of pyruvate to acetyl CoA. In this
reaction, one molecule each of the catalytic coenzymes TPP, FAD and lipoamide
participate in the reaction but are not consumed.
True
False
DIES
<< Previous
3 pt
ve Last checked at 10:08pm
Next
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- What is the function of glyceraldehyde 3-phosphate dehydrogenase? catalyzes the transfer of a phosphoryl group from ATP to an acceptor oxidation of the alcohol to an aldehyde dehydration and dephosphorylation of GAP hydrolysis of GAP oxidation by NAD+ and formation of acyl-phosphatearrow_forwardWhich of the following is true about comparing the α-ketoglutarate dehydrogenase complex and the pyruvate dehydrogenase complex? Group of answer choices They catalyze reactions that produce the exact same set of products They are both dehydrogenase complexes, but have very different structures They both catalyze reactions that oxidize NADH to NAD+ They both use the same E3 enzyme component Which of the following best describes stage 2 of the citric acid cycle? Group of answer choices ATP production by the electron transport chain production of acetyl CoA regeneration of oxaloacetate release of 2 carbons as carbon dioxidearrow_forwardConsider the reaction shown, which of the following conditions is most likely to lead to the activation of pyruvate dehydrogenase? high concentrations of acetyl-CoA high concentrations of NADH high concentrations of NAD+ high concentrations of CO2arrow_forward
- if each NADH generates 3 ATP molecules and each FADH2 generates 2 ATP molecules, calculate the number of ATP molecules generated from one saturated 10-carbon fatty acid Determine the number of repetitions of the β‑oxidation spiral needed to completely degrade the fatty acid to acetyl-SCoAacetyl-SCoA (acetyl-CoA)(acetyl-CoA) . Calculate the ATPATP produced by the acetyl-SCoAacetyl-SCoA molecules in the citric acid cycle. Calculate the ATPATP produced from the FADH2FADH2 and NADHNADH produced from β‑oxidation. Add the ATPATP generated (from step 2 and 3), and subtract the ATPATP needed to activate the fatty acid.arrow_forwardThe citric acid cycle is mainly controlled at the first two NADH-producing reactions catalyzed by ___________ and ___________, respectively. Group of answer choices isocitrate dehydrogenase; alpha-ketoglutarate dehydrogenase complex pyruvate dehydrogenase complex; alpha-ketoglutarate dehydrogenase complex alpha-ketoglutarate dehydrogenase complex; malate dehydrogenase isocitrate dehydrogenase; malate dehydrogenase pyruvate dehydrogenase complex; isocitrate dehydrogenasearrow_forwardHexokinase phosphohexose isomerase [Choose ] [Choose ] Is not the trapping reaction catalyzes an ATP-dependent phosphorylation of its substrate. Is irreversible and carries out substrate-level phosphorylation. PFK-1 aldolase triose-phosphate isomerase Splits the carbohydrate in two. Product is 2-phosphoglycerate. Is reversible and carries out substrate-level phosphorylation. Product is fructose-6-phosphate. Catalyzes the trapping reaction Makes a 2nd mole of G3P from DHAP Produces a high-free energy compound but no NADH or ATP. Oxidizes an aldehyde. GAPDH [Choose ] Phosphoglycerate kinase [Choose ]arrow_forward
- A patient exhibiting all the symptoms of beriberi is placed on a thiamine-enriched diet; however, the symptoms do not disappear. Genetic screening suggests a defect in pyruvate dehydrogenase phosphatase. Briefly explain why the genetic defect caused beriberi symptoms andy why thiamine supplementation had no effect.arrow_forwardThe pyruvate dehydrogenase complex catalyzes the synthesis of acetyl-CoA, a very important molecule in cellular metabolism. The reaction catalyzed by the pyruvate dehydrogenase enzyme complex is shown below. This reaction is tightly regulated, because it is essentially irreversible. Which of the following conditions is most likely to lead to the inhibition of pyruvate dehydrogenase in cells? (select two answers) High concentrations of acetyl-CoA High concentrations of pyruvate High concentrations of NADH High concentrations of NAD+ High concentrations of CoAarrow_forwardwhat type of regulator is Acetyl-CoA for each?arrow_forward
- Fill in the blanks with the numerical answer only: Number of electrons generated in oxidation of 1 glucose to 2 pyrvuate in glycolysis. Number of electrons generated in the oxidation of 2 pyruvate by pyruvate dehydrogenase complex. Number of electrons generated for complex II in oxidation of 2 acetyl CoA via the TCA Cycle.arrow_forwardcetyl CoA produced from pyruvate: * not depends on the coenzyme biotin. | occurs in the cytosol. involves the participation of lipoic acid. | all of the above is activated when pyruvate dehydrogenase (PDH, E1) of the pyruvate dehydrogenase stagel de lll 21.02.28arrow_forwardWhat are the coenzymes of El and E2 of pyruvate dehydrogenase complex? Explain their roles and reactions.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON