
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Give clear detailed Solution with explanation needed. Don't give Ai generated solution. Avoid handwritten Solution
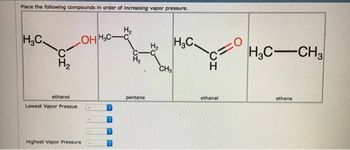
Transcribed Image Text:Place the following compounds in order of Increasing vapor pressure.
H.C.
C
H₂
OHHC-C
H₂
ethanol
Lowest Vapor Pressue
0
0
Highest Vapor Pressure
pentane
CH3
H3C.
H3C-CH3
ethanal
ethane
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- How do I approach this questionarrow_forwardOrder it! Lowest to Highest!! Based on intermolecular forces, predict the ordering from LOWEST boiling point to HIGHEST boiling point. CH3CH2CH2OH Ne CH3COCH3 CH4 CH3CH2CH3arrow_forwardThe predominant intermolecular force between molecules of the following compound is: H H-c-OH H-C-cH H. H.arrow_forward
- Give detailed Solution with explanation needed..don't give Handwritten answer...arrow_forwardBased solely on IMF, list the following substances from highest vapor pressure to lowest vapor pressure: CH3CH2OH , CH3CH2Br , CH3CH3 and BaCl2arrow_forward(e) lowest boiling point CH3CHCHCH3 OCH3CH₂CH3 OCH₂CH₂ Since this molecule is ---Select--- (f) highest boiling point HCI HBr HF Since this compound has ---Select--- (g) lowest vapor pressure at 25°C OCH3CH₂OCH₂CH3 CH3CH₂CH₂CH₂OH OCH3CH₂CH₂CH3 Since this compound has ---Select--- and ---Select--- ♥ than the other two, it has the ---Select--- ↑ ---Select--- unlike the other two compounds, it has the ---Select--- unlike the other two compounds, it has the ---Select--- and will have the lowest boiling point. intermolecular forces and will have the highest boiling point. intermolecular forces and will have the lowest vapor pressure.arrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн IIIII Н-С-С-С-С-С-Н ннннн O III>I>IV>I| © II>IV>I>III O III>I>II>IV IV>II>I>III © I>III>II>IV нннн н-с-с-с-с-о-н .... нннн || н НIН нн С н Н-С-С-С-С-Н H H H H H нн Ш Η Η Η Η н-с-с-с-C-N-H ..... НЕНН Н IVarrow_forwardWhich of the following pure substances will have hydrogen bonds? H CH3 CH3 CH3 CH3 H H (acetone) (dimethyl ether) (methanol) a. acetone and methanol b. dimethyl ether c. acetone d. methanolarrow_forwardPlace the following molecules in order from lowest boiling point to highest boiling point, according to their intermolecular forces. Briefly explain your choices, using specific IMF vocabulary.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning