
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Please help me fix this drawing!
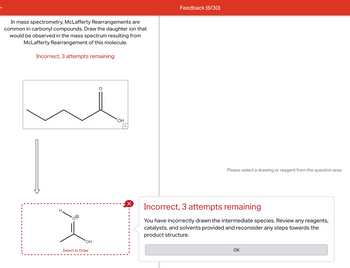
Transcribed Image Text:In mass spectrometry, McLafferty Rearrangements are
common in carbonyl compounds. Draw the daughter ion that
would be observed in the mass spectrum resulting from
McLafferty Rearrangement of this molecule.
Incorrect, 3 attempts remaining
H.
OH
Select to Draw
OH
Q
☑
I
Feedback (6/30)
Please select a drawing or reagent from the question area
Incorrect, 3 attempts remaining
You have incorrectly drawn the intermediate species. Review any reagents,
catalysts, and solvents provided and reconsider any steps towards the
product structure.
OK
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 1. Which one of the given compounds is consistent with the mass spectrum below? 100 40 20 10 20 25 30 35 40 5o 55 45 60 65 70 75 m/z Courtesy of SDBS: National Institute of Advanced Industrial Science and Technology A. CH,CH,CH(CH,), В. CH,CHOHCH,CH, C. CH,CH,OCH,CH, D. CH,CH,NHCH,CH, E. CH,CH,CH,CH, Relative htensityarrow_forwardMechanism ?arrow_forwardsub= 18 helparrow_forward
- What cations are formed in the mass spectrometer by α cleavage of each of the following compounds?arrow_forwardWhich one of the following statements is not true? O In mass spectrometry, the initially formed radical cation can sometimes survive and appear as a peak on the mass spe O Visible light is higher in energy than infrared light. O In mass spectrometry the peak corresponding to an ethyl cation fragment would appear at m/z 30. O In mass spectrometry, a benzylic cation would appear at m/z 91. O In IR spectroscopy, a C-O bond stretch would appear at a lower cm1 than a C=O bond stretch. %23 $ & 8 2 3 4 6 8 Q E R T. Y 4 5 2 K D G H. C || N M. altarrow_forwardIn mass spectrometry, alpha cleavages are common in molecules with heteroatoms. Draw the two daughter ions that would be observed in the mass spectrum resulting from an alpha cleavage of this molecule. Incorrect, 4 attempts remainingarrow_forward
- 7 Predict the masses and the structures of the most abundant fragments observed in the mass spectra of the followingcompounds. 4-methylpentan-2-olarrow_forwardNonearrow_forward8) In mass spectrometry (M.S.), the molecular ion (M) is usually the largest peak. For what type of compounds would you expect the (M-1)* peak be quite large. a) alkyl bromides c) alcohols e) alkyl chlorides b) aromatic compounds d) hydrocarbonsarrow_forward
- Why is it important for solutions to be diluted before they are run through spectroscopy?arrow_forwardModel 3: Ionization and Fragmentation Most mass spectrometers accelerate a molecule by first turning it into an ion, then using an electric field to accelerate it toward the detector. During standard ionization, a molecule loses one electron, the electron that is easiest to remove. The following is a hierarchy of electrons from easiest to hardest to remove: Electron in a lone pair (easiest) Electron that is part of a double bond (pi bond) Electron in a single bond (hardest) Knocking off an electron to make a +1 ion can be a harsh process. This harsh treatment often results in a broken bond, generating two smaller pieces, a +1 ion and a neutral fragment. (Note that only the ion is accelerated and detected.) ● 7. ● Critical Thinking Questions 6. If acetone is ionized, which electron is most likely to be knocked off? Replace this electron with a + (to indicate the missing electron) on the structure of acetone → The ion you drew above is called the "molecular ion" of acetone. It is…arrow_forwardWhich of the molecules below would be consistent with a compound having a (M)** of 102.0678 m/z in a high-resolution mass spectrum? CI NH2 NH2 Save for Later Submit Answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
